Abstract
Purpose
To determine how severe peripheral field loss (PFL) affects the dispersion of eye movements relative to the head, while walking in real environments. This information should help to better define the visual field and clearance requirements for head-mounted mobility visual aids.
Methods
Eye positions relative to the head were recorded in five retinitis pigmentosa patients with less than 15° of visual field and three normally-sighted people, each walking in varied environments for more than 30 minutes. The eye position recorder was made portable by modifying a head-mounted ISCAN system. Custom data processing was implemented to reject unreliable data. Sample standard deviations of eye position (dispersion) were compared across subject groups and environments.
Results
PFL patients exhibited narrower horizontal eye position dispersions than normally-sighted subjects (9.4° vs. 14.2°, p < 0.0001) and PFL patients’ vertical dispersions were smaller when walking indoors than outdoors (8.2° vs. 10.3°, p = 0.048).
Conclusions
When walking, the PFL patients did not increase their scanning eye movements to compensate for missing peripheral vision information. Their horizontal scanning was actually reduced, possibly because saccadic amplitude is limited by a lack of peripheral stimulation. The results suggest that a field-of-view as wide as 40° may be needed for closed (immersive) head-mounted mobility aids, while a much narrower display, perhaps as narrow as 20°, might be sufficient with an open design.
Introduction
Tunnel vision, or severe peripheral visual field constriction, impairs mobility due to difficulties in navigation and orientation and a reduced ability to spot obstacles. In most jurisdictions patients are considered legally blind if their residual visual field is less than 20° along the horizontal meridian. Diseases such as retinitis pigmentosa (RP), choroideremia, and advanced glaucoma can cause this type of peripheral field loss (PFL)1–3. About 2% of adults older than 40 suffer from glaucoma1,. An estimated 0.020% to 0.035% of individuals suffer from RP1, 4.
Many patients with severe PFL retain good visual acuity until an advanced stage of the disease; therefore, the main difficulty for these patients is gathering sufficient information about the environment for effective orientation and navigation5. A wider range of scanning eye positions in PFL patients than in normally-sighted people is presumed necessary both horizontally (to avoid lateral obstacles or detect objects approaching from the side) and vertically, particularly in the downward direction (following paths or traversing through uneven terrain, curbs, and stairs). Patients are frequently trained to systematically and quickly scan the environment to gain the information necessary for navigation, in the hope that the resulting increased dynamic field of view will compensate to some extent for the reduced static visual field5. To our knowledge, it has not been demonstrated that larger scanning amplitudes are actually performed following such training, or by such patients. Scanning might be achieved through eye movements alone or through a combination of eye and head movements. Eye movements are faster than head rotation and therefore might be more efficient. Thus, one might expect a wider dispersion of eye movements with severe PFL.
Turano et al.6 measured the direction of gaze (affected by both eye and head movements) of patients with severe PFL (due to RP) while walking for about 1 minute. The scanning strategies of the PFL patients differed from those of the normally-sighted subjects: the mean angular area scanned by the gaze point was approximately 10 deg² in the normally sighted group, but it was about three times larger for the PFL patients. Turano et al.6 did not report which part of the increased gaze point scanning was achieved through eye movements and which part through head movements. Knowledge of that division may help in designing and improving scanning training for patients with severe PFL.
In addition to recommending increased scanning, several optical (minifying) visual aids have been proposed for rehabilitation of patients with severe PFL7. Minifying aids for navigation include hand-held divergent lenses8 and reversed spectacle-mounted telescopes9–12. The loss of resolution inherent in minification and the interaction of the dynamic field of the patients with the field through the aid have been cited as reasons for rejection of these aids5, 13. To overcome these limitations we have proposed and implemented video-based augmented view systems using see-through head-mounted displays (HMD)14–16. Superimposing a low-resolution minified contour image over the residual visual field enables access to the wider field provided by the minification, while maintaining the full resolution of the natural view available through the display. The field of view through a mobility visual aid for a person with PFL should accommodate not only the patient’s residual visual field, but also the dynamic field that the patient typically would access. Otherwise, the mobility aid might restrict the effectiveness of the patient’s eye scanning movements. In designing an HMD, achieving a large field-of-view is a major challenge. Thus, a good design will have the smallest display field that satisfies the functional needs of a person with severe PFL. The field of the HMD can be scanned only with eye movements, while the environment seen through the display may be scanned with both eye and head movements.
The aim of this study was to measure the dispersion of eye positions in patients with severe PFL during independent mobility. We measured eye positions while walking for a long period (half an hour), in unfamiliar indoor and outdoor environments, including the many different visual tasks that such mobility entails. Eye movements were measured with reference to the head.
Materials and Methods
The study was conducted in accordance with the tenets of the Declaration of Helsinki.
Subjects
Five patients with severe PFL due to RP were recruited, and three normally-sighted people served as the control group. The inclusion criteria for patients were: less than 20° of total residual horizontal and vertical visual field extent in both eyes, visual acuity with refractive correction of better than 20/50, and good independent mobility skills. Subject characteristics are summarized in Table 1.
Table 1.
Subjects characteristics. #_RP denotes a PFL patient and #_NS denotes a normally-sighted subject. Field is the binocular field horizontal diameter derived from two monocular field measurements, VA specifies the visual acuity for each eye, Rx Correction indicates whether or not corrective lenses were worn.
| Subject # | Field (deg) | VA (OD; OS) | Age (years) | Rx Correction | Mobility aids |
|---|---|---|---|---|---|
| 1_RP | 10° | 20/30; 20/30 | 66 | Yes | Long cane |
| 2_RP | 12° | 20/20; 20/20 | 59 | Yes | Long cane |
| 3_RP | 15° | 20/30; 20/30 | 76 | No | Long cane |
| 4_RP | 7° | 20/30; 20/50 | 34 | No | Long cane, occasional |
| 5_RP | 11° | 20/20; 20/40 | 54 | No | None |
| 1_NS | No visual loss | 20/20; 20/20 | 73 | Yes | None |
| 2_NS | No visual loss | 20/20; 20/20 | 64 | Yes | None |
| 3_NS | No visual loss | 20/25; 20/30 | 66 | Yes | None |
Vision measures
Monocular visual field extent in both eyes was measured using an AutoPlot perimeter (Bausch & Lomb, Rochester, NY), in a dim room, with a 6mm white light target on a whiteboard at a distance of 1m. Monocular visual acuity in both eyes was measured using the B-VAT II SG (Mentor O&O, Norwell, MA).
Eye tracking equipment
A modified ISCAN® head-mounted eye tracking setup with an RK426-PC PC Board (ISCAN, Inc., Burlington, MA) was used. An infrared (IR) video camera recorded eye pupil position relative to the head-mounted camera, while a second camera recorded the scene. The scene camera, approximately optically conjugated with the eye pupil, was used in this study only for calibration purposes and to determine the walking environment in each segment during analysis. The video signals from the pupil and scene cameras were recorded with two small Canon ZR10 digital camcorders that served as video cassette recorders (VCRs). Subjects carried the camcorders, the batteries, and the camera driver in a small shoulder bag.
In normal use, the ISCAN’s pupil camera directly feeds the RK426-PC PC Board that performs a dark-pupil tracking algorithm. Here, following the walking session, the recorded video was fed into the board. Each video field was sampled into 512(H)×256(V) pixels every 16.7msec, and was processed to estimate pupil center position (h,v), pupil diameter, and corneal reflection location.
To keep the ISCAN stable on the head during walking, we replaced the original headband with an adjustable head mount from an indirect ophthalmoscope. In addition, some subjects wore a silicone swimming-cap under the head-mounted apparatus to provide improved friction and stability17. This system interfered minimally with the normal view of the subjects, enabling use of habitual eye and head scanning, and could be worn without discomfort for extended periods while walking.
Eye movement recording procedure
The subjects each walked independently for more than 30 minutes in total, using their normal visual refractive correction and long canes. They performed free eye (and head) movements during their navigation, and received no instructions other than to walk normally to various points along a route. Only walking directions to the next point were given. The environments were not controlled for either numbers of pedestrians crossing or obstacles. Subjects were not assisted; they located and opened doors, pressed elevator buttons, etc. The route was similar, but not identical, for all subjects due to the effects of weather, sun position, and building and sidewalk construction. The route included segments in unfamiliar indoor environments (including stairs, ramps, closed doors, elevators, and parking garage, in either a hospital or government office building) and city streets (including traffic, pedestrian crossings, and naturally occurring obstacles). Subjects spent several minutes in each environment (9 minutes on average). Since walking speed was not dictated and the route was not exactly the same for all subjects (as explained above), the length of time for each subject in each environment varied (see Table 2 for recording duration data). Due to recording artifacts, data from most segments were not processed in their entirety.
Table 2.
Eye position dispersion in degrees, horizontal (SH) and vertical (SV), for each subject and environmental segment, recording time, number of useful data samples, and percentage of useful samples for each segment.
| Subject | Environment | SH (deg) | SV (deg) | Time (min.) | Valid samples (%) | |
|---|---|---|---|---|---|---|
| Control Subjects | 1_NS | Indoor | 16.16 | 8.10 | 5.01 | 91 |
| Indoor | 14.39 | 9.99 | 5.74 | 88 | ||
| Outdoor | 13.84 | 9.97 | 7.93 | 87 | ||
| Indoor | 14.74 | 9.44 | 5.73 | 86 | ||
| Outdoor | 8.95 | 10.73 | 8.45 | 84 | ||
| 2_NS | Indoor | 14.08 | 13.41 | 5.69 | 90 | |
| Outdoor | 12.73 | 6.96 | 6.80 | 89 | ||
| Indoor | 14.34 | 5.93 | 6.27 | 92 | ||
| Outdoor | 13.84 | 8.33 | 9.66 | 86 | ||
| Indoor | 13.23 | 7.99 | 1.99 | 90 | ||
| 3_NS | Arcade | 18.58 | 11.68 | 13.81 | 26 | |
| Outdoor | 16.50 | 11.07 | 11.10 | 29 | ||
| PFL patients | 1_RP | Arcade | 9.44 | 7.27 | 26.50 | 74 |
| 2_RP | Outdoor | 8.71 | 10.31 | 5.05 | 91 | |
| Indoor | 8.24 | 5.84 | 3.61 | 92 | ||
| Indoor | 8.68 | 8.16 | 4.88 | 96 | ||
| Arcade | 8.56 | 10.99 | 18.37 | 60 | ||
| Arcade | 10.38 | 9.73 | 22.24 | 41 | ||
| 3_RP | Arcade | 10.38 | 9.73 | 14.21 | 70 | |
| Outdoor | 10.33 | 11.16 | 13.82 | 11 | ||
| Outdoor | 7.71 | 5.91 | 5.50 | 8 | ||
| Indoor | 10.70 | 4.98 | 3.84 | 58 | ||
| 4_RP | Indoor | 9.28 | 7.96 | 13.52 | 88 | |
| Indoor | 8.48 | 9.91 | 5.22 | 89 | ||
| Outdoor | 9.65 | 10.41 | 10.96 | 48 | ||
| 5_RP | Indoor | 12.73 | 8.71 | 10.69 | 71 | |
| Outdoor | 10.66 | 10.35 | 10.17 | 47 | ||
| Indoor | 11.86 | 9.26 | 2.63 | 73 |
We tried to avoid bright sunny spots that caused extended corneal reflections and resulted in failed detection of pupil position. Light blockers, such as sunglasses and tinted visors, were ineffective, and some patients rejected them. Therefore, cloudy days and late afternoons were preferred for the outdoor walking. When the sun caused difficulties with eye tracking on a scheduled walk, the route was redirected where possible under arcades (covered passageways) in the shade of buildings, resulting in an outdoor environment that had some of the characteristics of the indoor environments. These segments were named “arcades” and were scored separately. Following the whole recording session, subjects were debriefed about the strategies they generally followed when walking and navigating through environments similar to ones walked in the study.
Each time a subject entered a new segment of the route, angular calibration recordings were made, so that any shifts of the headgear could be determined. We used a 3x3 grid of fixation targets (spanning about 18°) on a frame mounted with a lightweight boom (32 cm) on a portable bite bar with a customized dental imprint that assured stable and repeatable positioning. Eye positions were recorded with subjects fixating monocularly for a few seconds on each of the 9 points.
A calibration procedure was carried out in the lab to determine the angle equivalence (α*, β*)m=1…9 of the 9 calibration targets for each subject. We used either a picture of the calibration frame taken with the scene camera including known angular calibration markers, or a perimeter monocular measurement of the subtended angle for each fixation point.
Data Processing
The first stage in data processing was to exclude erroneous data. The dark-pupil tracker sometimes failed to deal either with bright sunlight reflections off the cornea, or when a large part of the image was as dark as the pupil. In addition, blinks, sudden illumination changes, and shadows could cause the instantaneous estimates of pupil position to be inaccurate. However, using appropriate settings, the ISCAN was able to provide acceptable tracking for most of the frames.
We developed an automatic algorithm to discard erroneous frames, allowing the processing of large amounts of data with minimal operator intervention. First, we imposed limits on the acceptable horizontal and vertical position coordinates of the pupil center. The operator set these limits after reviewing the recorded video. Second, unreasonable pupil diameter values were used to detect erroneous eye position data. Since the normal pupil dimensions changed along the walking route due to the variations in illumination, we imposed an adaptive rather than a fixed restriction on the pupil diameter value. Figure 1 shows an actual plot of this adaptive pupil diameter selection. For each frame we calculated a running average pupil diameter based on a temporal window of 1000 frames (16.67 sec.) centered on the evaluated frame. We rejected those frame data in which the pupil diameter was outside a range of ±200 ISCAN units from the average pupil diameter.
Figure 1.
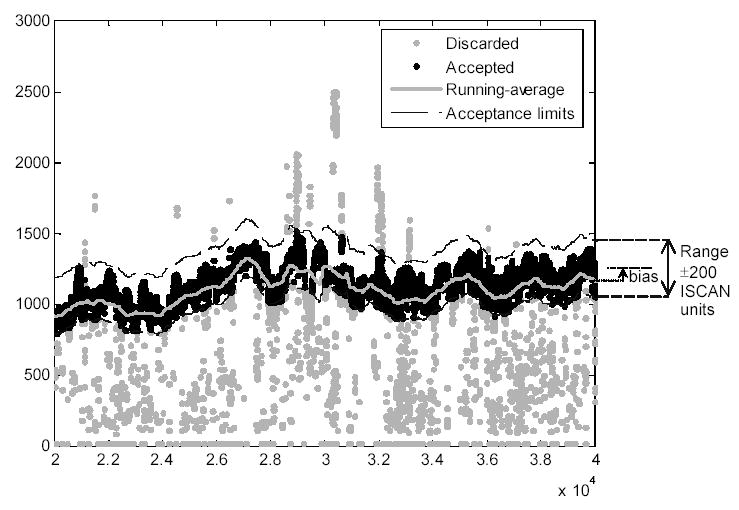
Frame rejection criteria based on pupil diameter. The graphic illustrates the pupil diameters obtained from the ISCAN in each frame during a time segment of approximately 5.5 minutes (each dot represents one data point). The continuous gray line in the middle of the black data represents the running-averaged pupil diameter, and the black dashed lines on the right show the allowed pupil diameter range. The accepted frames (88%) are displayed as black symbols and the discarded ones as gray symbols. The acceptance band is set by the window size (in this case ±200 ISCAN units) centered on the bias-corrected running average.
Additional corrections were also applied:
A slight bias or offset (arbitrarily selected up to 75 units) from the average signal towards the larger pupil values was introduced. This compensated for the fact that small pupil diameter values were more frequent in the unreliable frames. Therefore the running-average diameter was, in these cases, smaller than the expected mean pupil diameter in the reliable frames.
We also imposed a minimum for the running-average pupil diameter (100 units) to reject those segments in which most of the frames were not useful (associated with very small pupil diameter estimates). The percent of valid data obtained following all these procedures is reported in Table 2.
After eliminating erroneous data, valid data were converted from raw ISCAN units to angular eye rotations. The raw units (h,v) used internally by ISCAN for pupil position form a 5120×2560 matrix representing sub-pixel resolution. Each pixel of the eye camera corresponds to approximately 48μm at the pupil plane (10 horizontal and 5 vertical ISCAN units). To convert pupil pixel coordinates (h, v), into angular horizontal and vertical rotation of the eye (α, β), expressed in degrees, we arbitrarily used a quadratic polynomial expression:
| Equation 1 |
where (a,b)ij are the five pairs of polynomial coefficients obtained from the 9-point calibration grid.
To derive the conversion coefficients the angular coordinates (α*, β*)m of the m-th calibration grid point (computed from the known grid size and distance from the observer measured in the lab calibration procedure) was fitted to the averaged pupil position (hm,vm) measured during fixation on each of the calibration targets. Using a least squares algorithm for bi-dimensional second order polynomial fitting in MATLAB, we obtained the coefficients (a,b)ij :
| Equation 2 |
Characterization of angular eye position dispersion
The entire recorded video from each subject was divided into segments corresponding to walks in specific environments. Each segment was preceded by a calibration recording. Each segment was assigned to one of three environment classes: indoors, outdoors, or arcades, based on the images provided from the scene camera in the post-processing. We characterized the eye position dispersion for each segment using the sample horizontal and vertical standard deviations (SH ,SV referred to as ‘dispersions’) of the angular eye positions (α, β). Dispersion provides a measure of the range of eye movements during walking and thus provides a guide to the size of the field-of-view of a head mounted device that might be useful as an aid for a person with severe PFL.
Statistical analyses
To determine if normal distributions could be assumed, Lilliefors two-sided normality tests were performed on the distributions of eye position (α, β), and the distributions of angular dispersion (SH , SV) of each group (normally sighted and PFL) in each environment, for horizontal and vertical components separately. The hypothesis of normality was rejected for all the eye position distributions. Nevertheless, the dispersions computed as standard deviations are mathematically defined for any distribution. As one sample of angular dispersions failed the normality test, we used a Wilcoxon two-sided rank sum test for all comparisons of the dispersions between groups and environments. All the analyses were performed using the Statistics Toolbox (Version 5.0.1) of MATLAB® R14SP1 (The MathWorks Inc., Natick, MA).
Results
Figure 2 shows examples of bi-dimensional histograms of vertical and horizontal eye positions for one normally-sighted and one RP patient with PFL, for indoor and outdoor segments. Values were binned in squares of 2° × 2°. The ordinates indicate the percent of valid frames for which the eye was within each bin area. These histograms were selected because they present a smooth bell shape. The horizontal extent of the eye position dispersion for the PFL patient is noticeably narrower than that for the normally-sighted subject. When walking outdoors, the PFL patient scanned over a larger vertical range than when indoors. The other histograms (not included) showed the same general patterns of eye position dispersions; however, some were not as smooth as the ones presented here.
Figure 2.
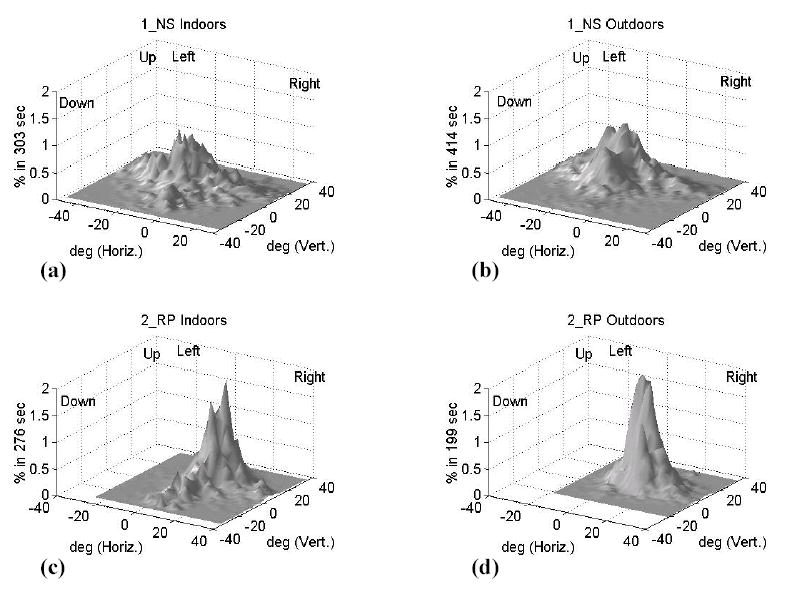
Examples of bi-dimensional histograms of vertical and horizontal eye position for two subjects and two conditions: a) normally sighted subject (1_NS) indoors and b) outdoors; c) PFL patient (2_RP) indoors, and d) outdoors. Note the change in pattern for the PFL patient between the two environments. Ordinates indicate the percent of valid frames for which the eye was within each bin area. The spatial position on the abscissas is relative to the central fixation point on the portable calibration grid.
Table 2 lists the results for each subject and segment chronologically, excluding the calibration segments and totally rejected segments. The “valid samples %” columns specify the percent of accepted video frames in the segment with respect to the total number of frames of the recorded segment. This percentage provides an indication of the quality of the eye-tracking in that segment. As can be seen, many of the segments yielded about 90% valid data. The segments where the percent valid data was much lower were usually associated with bright outdoor weather conditions impeding the recording.
Figure 3 shows a scatter plot representation of horizontal (SH) and vertical (SV) dispersions, where each point represents the collapsed data for all segments of each subject within each environment. In general, for PFL patient segments (represented as open symbols) the horizontal eye position dispersion is narrower than for normally-sighted subjects (solid symbols). It is also apparent, for the patients with PFL, that the vertical dispersion outdoors (open triangles) is larger than the vertical dispersion indoors (open squares).
Figure 3.
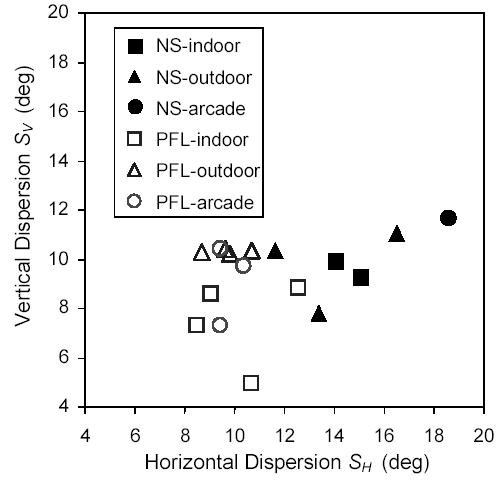
Horizontal and vertical eye position dispersions (standard deviations) for normally-sighted subjects (NS) and PFL patients. Each symbol corresponds to the data for a subject in a particular environment (indoors, outdoors, or arcades) collapsed over all segments.
In Figure 4, box plots show the horizontal (SH) and vertical (SV) dispersions obtained in the segments (as reported in Table 2), across groups (normally sighted and PFL) and for the relevant environments (indoors, outdoors, and total accumulation of segments including arcades). We have excluded segments categorized as arcades from the comparisons, because we considered them as weather-forced situations that did not exactly match either of the other conditions. Wilcoxon two-sided rank sum testing for identical populations revealed no significant differences (p > 0.2) between indoor and outdoor dispersions in normally-sighted subjects, or the indoor and outdoor horizontal component for PFL patients, so we only present the collapsed totals for those conditions.
Figure 4.
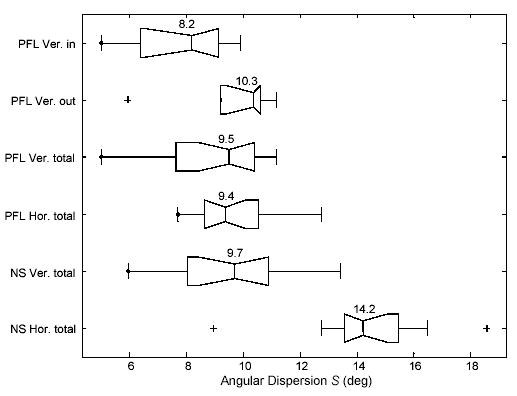
Box plots of angular eye position dispersion in normally-sighted subjects (NS) and PFL patients. Data are segmented by environmental condition: indoors (in), outdoors (out) and overall (total of in and out); and by dispersion component: vertical (Ver.) and horizontal (Hor.). Values for the sample medians and quartiles are displayed in the boxes and the whiskers represent the rest of the sample (unless there are outliers). The few “+” symbols represent outliers, values more than 1.5 times the interquartile range away from the top or bottom of the box.
Normally-sighted subjects demonstrated a wider horizontal than vertical dispersion (p = 0.0003). However, in PFL patients, vertical and horizontal dispersions were similar. Furthermore, both were equivalent to the normally-sighted subjects' vertical dispersion. PFL patients demonstrated a narrower horizontal dispersion of eye positions compared to the normally-sighted subjects; this was true for all environmental conditions (p < 0.0001). Although we expected to find a difference between groups, this result is the opposite of our expectation. Within the PFL group, we did not find any correlation between the residual horizontal visual field extent and eye position dispersion.
As seen in Figure 3, PFL patients behaved slightly differently outdoors and indoors. Patients increased their vertical scanning dispersion while walking outdoors (p = 0.048) compared to walking indoors, as we would expect from their need to monitor obstacles or irregularities at ground level. We have evaluated whether the increase in vertical dispersion when outdoors occurred mainly toward the lower field. For the three subjects who performed both indoor and outdoor walks, two had a slight expansion of the distribution downward (as was shown in the example in Figure 2d), but the main effect was an overall (symmetric) increase in vertical dispersion.
Discussion
Since we are developing field expansion devices based on head-mounted displays (HMD), we measured the dispersion of eye movements relative to the head. We believe that this measure could help us define the necessary field-of-view to be used in such displays. A wide field-of-view is one of the most difficult parameters to achieve in HMD design and is usually associated with loss of resolution18. We also hoped to gain a better understanding of the variables that affect eye movements in the face of the challenges imposed by severe PFL (tunnel vision) when walking.
Our findings with normally-sighted subjects (dispersion of 9.7° vertically and 14.2° horizontally) are comparable with the finding of a previous study conducted under similar conditions19. Bahill et al.19 used electro-oculography (EOG) and reported only the distribution of saccade amplitude during extended walking on a college campus. They found that most saccades measured less than 15°. For the PFL patients, we found dispersion values of 9.5° in the vertical direction and 9.4° for the horizontal (about 2/3 that of the normally sighted). Despite the small sample size, the difference in horizontal dispersion between PFL patients and normally-sighted subjects was found to be statistically significant.
Other studies of the effects of restricted visual fields on eye movements have been carried out in different contexts. Turano et al’s6 recording methodology was similar to ours, but they measured only a few seconds of indoor walking, and analyzed mainly gaze position, identifying objects that were fixated. Other studies focused on gaze movements (meaning compound eye + head position) during search tasks on displays; either by normally-sighted people with simulated PFL20, or by PFL patients using an augmented viewing device16.
The implication from our results is that head-mounted mobility visual aids for severe PFL may be effective even with a relatively narrow field-of-view. A field-of-view covering four times the dispersion found would keep about 96% of fixations inside the active area of the visual aid, if eye fixations are assumed to be normally distributed. Consequently, desirable fields would be about 40°×40°. Immersive HMDs prevent any vision outside the active display area and may indeed require a field-of-view that wide. The required field might be even wider if the full extent of the residual field is to be within the display all the time. On the other hand, an open display design permits natural viewing outside the active area of the display. Therefore, to provide augmented visual information, the field-of-view of an HMD – to be implemented in a mobility visual aid for PFL – need not be more than double the eye position dispersion found for the PFL group. Hence, displays that subtend about 20° both horizontally and vertically might be sufficient. Such a display assures that, most of the time (about 64%), the line of sight of users with PFL will be within the active display field, providing useful information. Manufacturers of HMD-based magnifying low vision aids generally seek wider fields of view. However, displays with a narrower field-of-view available at a lower price would make potential aids for PFL more affordable. Smaller displays should help PFL patients locate their targets of interest within the display itself, since, in most cases, they would not have to scan the active area. With a smaller display, the patients would have less trouble identifying their own gaze location within the display. This self-localization helps them achieve a quicker correspondence with the target location in the real environment. Luo and Peli16 proposed the inclusion of guide grids in the active area of an HMD to help patients locate the center of the display. A similar difficulty in self-locating within a display field was noted for simulated PFL in a visual search experiment with targets presented on computer monitors20.
We found a relative change in the vertical scan dispersion between indoor and outdoor environments for all PFL patients. This behavior is probably associated with differences in the navigation and obstacle-avoidance demands of the two environments. A patient walking indoors might expect to find an even floor, with little concern for low-lying obstacles or abrupt changes in elevation. On the other hand, walking outdoors entails increased concern for ground-level obstacles (e.g. uneven pavement and curbs) and head-level obstacles (e.g. low tree branches). This would require less attention to the floor and a consequent reduction in the vertical dispersion indoors. Most of our PFL patients used long canes to monitor the ground for obstacles and uneven pavement. One would expect that a patient who does not use a long cane might exhibit an even wider dispersion of vertical eye movements in outdoor mobility than we have found. Indoor mobility comes with an increased need to locate orientation features such as doors and hallways, as shown by Turano et al.6. However, the horizontal angular span of such features is limited by the structure of the corridors. While outdoors, objects located at more distant lateral locations are used mostly for navigation rather than safe mobility (obstacle avoidance) and they can usually be spotted from a farther distance where their angular span is limited. Yet the normally sighted subjects exhibited horizontal dispersions that were wider than their vertical dispersions. Possible reasons for this are discussed below. These differences suggest that while a display with wider horizontal than vertical field (landscape mode) might be better for normally sighted users, a square or even a portrait mode display with wider vertical span might be more useful for PFL patients, especially outdoors. For two of the three PFL subjects, who completed indoor and outdoor walks with a long cane, we found a trend toward an expansion of the vertical dispersion downwards when outdoors (though the main effect was a symmetrical expansion). Patients who do not use long canes may tend to vertically scan even more downwards. Thus, we suggest that an asymmetric setting of the display relative to the camera, using more of the display to cover the lower than upper field, might also be beneficial6.
The limited size for the display field-of-view discussed above does not imply that patients with PFL never use larger eye movements. In fact, we have found that they do perform occasional large scanning eye movements. Therefore, such visual aids should neither restrict nor block normal eye movements, nor restrict the dynamic visual field5. Moreover, since the severely restricted visual field of patients with PFL can be fully blocked by a small obstruction, the design of such displays and their field of fixation21, 22 should avoid even small peripheral obstructions (5 – 10°); therefore, immersive HMDs should be avoided for mobility applications. The term “clearance” of a visual aid, defined as the overall unblocked visual field, was adopted to indicate the field-of-view that allows unrestricted eye scanning by the user both inside and outside of the active display area of the visual aid15. As an example, some studies reported that PFL patients found the field of the Multi-Vision Night Vision goggles restrictive, even though it has a relatively wide display field of 32×24° 23–25. The concept of clearance suggests that a narrow display is more likely to be useful if it is embedded within a clear carrier lens, which is one of the implementations we have proposed15 and implemented16. Clearance of an HMD is not dissimilar to the peripheral field that is available to spectacle wearers outside of the rim of the frame, providing a non-optically corrected, yet important area of visual field.
A plausible explanation for our main finding of reduced horizontal eye position dispersions in patients with severe PFL is that head movements play an important role in scanning. This needs to be confirmed in future studies recording eye and head movements under similar conditions. PFL patients may have a better sense of direction when they use their body heading as their main reference. Some of the subjects reported such a strategy when debriefed. They reported selecting a reference or landmark directly ahead in the direction they were walking and trying to keep that landmark in view, only briefly shifting their gaze to scan the path between them and the landmark or to check for likely sources of hazards. They indicated that it was quite difficult to recover their landmark if their gaze had been shifted away by scanning eye movements. By comparison, scanning with head movements seemed to facilitate recovery of the primary position of gaze and facilitate regaining of the landmark.
Patients with PFL might use a wider scanning strategy in some situations, although on average they show a reduced dispersion of eye position while walking. A wide scanning strategy might be used at a critical street crossing or when searching for a misplaced object. Such situations may be observed more readily by rehabilitation personnel, which might explain the clinical impression of increased scanning by these patients.
For normally-sighted observers, saccades are usually aimed at some peripheral visual target. It is possible that the PFL patient would not make eye movements aimed anywhere outside of their field due to lack of peripheral stimulation and therefore would have a restricted range of eye movements; this might be the main reason for our finding. This concept of saccade inhibition is implicit in the results of Luo and Peli16. They showed that both PFL patients and subjects with simulated PFL reduced search time and increased directness of the gaze path to a target placed outside of the visual field, when using either auditory clues or an augmented view aid. The augmented viewing aid and auditory cues provided the missing peripheral information required to induce larger gaze movements towards the target stimulus. Cornelissen et al.20 also speculated about the effect of PFL limiting the ability to program efficient eye movements. Furthermore, there is evidence from reading eye movements in patients with hemianopia, that the lack of peripheral stimulation reduces saccade length; right hemianopes generally make smaller amplitude saccades to the right along a line than do normally sighted, and left hemianopes make many small amplitude leftward saccades when returning to the beginning of the next line rather than the single large leftward return sweep saccade made by normally sighted readers26, 27. A possibly related effect was described by Hassan et al.28, who showed that a moderate reduction of the visual field due to glaucoma had an impact on head movement patterns while crossing streets; the glaucoma patients did not exhibit head movements consistent with maximizing safety. The authors hypothesized that this result could be due to the dynamic nature and complexity of the street-crossing task. However, it is possible instead to advance an explanation based on the lack of visual stimulation from the peripheral field. Important objects such as oncoming cars were not noted and therefore did not induce shifting of the gaze to the blind field.
Thus, we believe that the absence of visual stimulation is probably the main cause for our primary finding of reduced horizontal ocular scanning in people with severe PFL. If this were the case, we might expect to find that horizontal dispersion would decrease as field size decreased; however, we did not find such a correlation, presumably because the sample size and range of visual field sizes examined were too limited. On the other hand, the strategy reported by patients of using proprioceptively-cued head movements to restore context would also account for the narrower eye movement dispersion found and need not be related to field size.
Acknowledgments
M.A. García-Pérez and members of Peli Lab for suggestions; A. Nugent for helping in data collection and analysis.
The Low Vision Research Group, in the ARVO 2001 Annual Meeting, awarded author F. Vargas-Martín the Constance Atwell Award for a poster presenting part of this work
This work was supported in part by NIH EYO5957, and EY12890 grants, and by grant FIS–PI021829 from Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Spain). F. Vargas-Martín also received a travel grant from Fundación Seneca, (Murcia, Spain).
Footnotes
Grants:
NIH EYO5957 and EY12890 grants, and FIS–PI021829 grant from Instituto de Salud Carlos III (Ministerio de Sanidad y Consumo, Spain).
F. Vargas-Martín has also received a travel grant from Fundación Seneca, (Murcia, Spain)
Declaration of Interests:
E. Peli has patent rights for some of the related technology; he has consulting and research funding with MicroOptical Corp. (Westwood, MA, USA).
References
- 1.Robinson B, Acorn CJ, Millar CC, Lyle WM. The prevalence of selected ocular diseases and conditions. Optom Vis Sci. 1997;74:79–91. doi: 10.1097/00006324-199702000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2803–2809. [PubMed] [Google Scholar]
- 3.Turano KA, Geruschat DR, Stahl JW, Massof RW. Perceived visual ability for independent mobility in persons with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1999;40:865–877. [PubMed] [Google Scholar]
- 4.Pagon RA. Retinitis pigmentosa. Surv Ophthalmol. 1988;33:137–177. doi: 10.1016/0039-6257(88)90085-9. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson C. Oxford: Butterworth-Heinemann; 1998. Low Vision: Principles and Practice. [Google Scholar]
- 6.Turano KA, Geruschat DR, Baker FH, Stahl JW, Shapiro MD. Direction of Gaze while Walking a Simple Route: Persons with Normal Vision and Persons with Retinitis Pigmentosa. Optom Vis Sci. 2001;78:657–666. doi: 10.1097/00006324-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JM. An overview of enhancement techniques for peripheral field loss. J Am Optom Assoc. 1993;64:60–70. [PubMed] [Google Scholar]
- 8.Kozlowski JM, Mainster MA, Avila MP. Negative-lens field expander for patients with concentric field constriction. Arch Ophthalmol. 1984;102:1182–1184. doi: 10.1001/archopht.1984.01040030960025. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy WL, Rosten JG, Young LM, Ciuffreda KJ, Levin MI. A field expander for patients with retinitis pigmentosa: a clinical study. Am J Optom Physiol Opt. 1977;54:744–755. doi: 10.1097/00006324-197711000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Krefman RA. Reversed telescopes on visual efficiency scores in field-restricted patients. Am J Optom Physiol Opt. 1981;58:159–162. doi: 10.1097/00006324-198102000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Hoeft WW, Feinbloom W, Brilliant R, et al. Amorphic lenses: a mobility aid for patients with retinitis pigmentosa. Am J Optom Physiol Opt. 1985;62:142–148. [PubMed] [Google Scholar]
- 12.Vargas-Martin F. Survey of a free cost viewfinder for visual field expansion. International Congress Series, Vision 2005 - Proceedings of the International Congress held between 4 and 7 April 2005 in London, UK. 2005;1282:1080–1084. [Google Scholar]
- 13.Fasce F, Bettin P, Lucà G, Brancato R. Effects of Minification on Visual Performance in Advance Glaucoma. In: Stuen C, Arditi A, Horowitz A, Lang MA, Rosenthal B, Seidman K, editors. Vision Rehabilitation: Assessment, Intervention and Outcomes. Lisse: Swets & Zeitlinger. 2000. pp. 177–179. [Google Scholar]
- 14.Peli E. Augmented Vision for Central Scotoma and Peripheral Field Loss. In: Stuen C, Arditi A, Horowitz A, Lang MA, Rosenthal B, Seidman K, editors. Vision Rehabilitation: Assessment, Intervention and Outcomes. Lisse: Swets & Zeitlinger. 2000. pp. 70–74. [Google Scholar]
- 15.Vargas-Martin F, Peli E. Augmented-view for restricted visual field: multiple device implementations. Optom Vis Sci. 2002;79:715–723. doi: 10.1097/00006324-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Luo G, Peli E. Investigative Ophthalmology & Visual Science. Use of an augmentedvision device for visual search by patients with tunnel vision2006 doi: 10.1167/iovs.05-1672. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turano KA, Geruschat DR, Baker FH. Oculomotor strategies for the direction of gaze tested with a real-world activity. Vision Res. 2003;43:333–346. doi: 10.1016/s0042-6989(02)00498-4. [DOI] [PubMed] [Google Scholar]
- 18.Piantanida T, Bowman DK, Larimer J, Gille J, Reed C. Studies of the field of view/resolution trade-off in virtual-reality systems. In: Rogowitz BE, editor. Human Vision, Visual Processing and Digital Display III/Depth Perception and Sensory Integration for Complex Visual Environments. San Jose, CA: The International Society for Optical Engineering (SPIE); 1992. pp. 448–456. [Google Scholar]
- 19.Bahill AT, Adler D, Stark L. Most naturally occurring human saccades have magnitudes of 15 degrees or less. Investigative Ophthalmology. 1975;14:468–469. [PubMed] [Google Scholar]
- 20.Cornelissen FW, Bruin KJ, Kooijman AC. The influence of artificial scotomas on eye movements during visual search. Optom Vis Sci. 2005;82:27–35. [PubMed] [Google Scholar]
- 21.Woods RL, Fetchenheuer I, Vargas-Martin F, Peli E. The Impact of Non-Immersive Head-Mounted Displays (HMD) on the Visual Field. Journal of the Society for Information Display. 2003;11:191–198. [Google Scholar]
- 22.Bailey IL. Critical view of an ocular telephoto system. Contact Lens Association of Ophthalmologists Journal. 1987;13:217–221. [PubMed] [Google Scholar]
- 23.Spandau UH, Wechsler S, Blankenagel A. Testing night vision goggles in a dark outside environment. Optom Vis Sci. 2002;79:39–45. doi: 10.1097/00006324-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Hartong DT, Jorritsma FF, Neve JJ, Melis-Dankers BJ, Kooijman AC. Improved mobility and independence of night-blind people using night-vision goggles. Invest Ophthalmol Vis Sci. 2004;45:1725–1731. doi: 10.1167/iovs.03-1061. [DOI] [PubMed] [Google Scholar]
- 25.Zebehazy K, Zimmerman G, Bowers A, Peli E. Establishing the reliability of mobility measures to assess the effectiveness of night vision devices: Results of a pilot study. Journal of Visual Impairment & Blindness. 2005;99:663–6670. [PMC free article] [PubMed] [Google Scholar]
- 26.Zihl J. Visual scanning behavior in patients with homonymous hemianopia. Neuropsychologia. 1995;33:287–303. doi: 10.1016/0028-3932(94)00119-a. [DOI] [PubMed] [Google Scholar]
- 27.Trauzettel-Klosinski S, Brendler K. Eye movements in reading with hemianopic field defects: the significance of clinical parameters. Graefes Arch Clin Exp Ophthalmol. 1998;236:91–102. doi: 10.1007/s004170050048. [DOI] [PubMed] [Google Scholar]
- 28.Hassan SE, Geruschat DR, Turano KA. Head movements while crossing streets: effect of vision impairment. Optom Vis Sci. 2005;82:18–26. [PubMed] [Google Scholar]


