Abstract
Superior colliculus (SC)–mediated control of visuomotor behavior depends on neuronal selectivity for stimulus velocity. However, the mechanism responsible for velocity tuning in SC neurons is unclear. It was shown in a previous study of anesthetized, decorticate hamsters that the number and distribution of feed-forward retinal inputs are not critical for velocity tuning. Here the alternate hypothesis that inhibition from the surround determines velocity tuning of SC neurons was tested. Surround inhibition was present in 65% (43/66) of SC neurons recorded in the superficial gray layer. Neurons within this group that were selective for slowly moving stimuli exhibited spatially asymmetric surround inhibition, and their velocity tuning arose by preferential suppression of responses to rapidly moving stimuli. In the other 35% (23/66) of SC neurons recorded, surround inhibition was weak or absent and did not play a role in velocity tuning. Most neurons with surround inhibition were nonselective for the duration of stationary flashed stimuli, whereas neurons without surround inhibition were selective for stimulus duration. The majority of neurons that preferred intermediate or rapidly moving stimuli exhibited spatially symmetric surround inhibition. In these neurons, occluding the surround reduced velocity selectivity by enhancing responses to slowly moving stimuli. Based on these data, a model is proposed suggesting spatiotemporal interactions between inhibition and excitation that could underlie velocity tuning.
INTRODUCTION
Velocity tuning is an essential component of image processing in many visual areas (Chalupa and Rhoades 1977; Cheng et al. 1994; Goodwin and Henry 1978; Hamasaki and Cohen 1977; Hess and Wolters 1979; Maunsell and Van Essen 1983; Movshon 1975; Orban et al. 1981; Stein and Dixon 1979; Tiao and Blakemore 1976), yet the underlying neural mechanism remains unclear (Liu and Newsome 2003). We have used the hamster retinocollicular system in our approach to understanding velocity tuning because of its advantages as a model system. The superior colliculus (SC) is an essential component in the motion processing and gaze-control pathway of all vertebrates. Its connectivity, circuitry, and response properties have been well described in rodents, cats, and monkeys (Huerta and Harting 1984; McIlwain 1991; Sparks 1999; Wallace et al. 1998; Wurtz and Optican 1994). Furthermore, the hamster SC provides an ideal system in which to investigate the development and plasticity of receptive field properties because hamster pups are born at a very early stage of brain development.
Velocity tuning could arise from feed-forward connections or from intrinsic circuitry within the target region (for review see Ferster and Miller 2000; Shapley et al. 2003). The relative contributions of these circuit components to velocity tuning in the superior colliculus are not known. The hamster retinocollicular system is uniquely suited for addressing this question because both feed-forward excitatory convergence and lateral inhibition can be manipulated independently. In a previous study we demonstrated that experimentally increasing the excitatory convergence of retinal ganglion cells onto SC neurons through postnatal blockade of N-methyl-D-aspartate (NMDA) receptors had no effect on velocity tuning, despite increases in receptive field (RF) size (Razak et al. 2003). This finding suggests that the feed-forward connectivity pattern does not play a significant role in shaping velocity tuning of SC neurons. Thus in this study we tested the alternate hypothesis that surround inhibition plays a prominent role in shaping velocity tuning in the SC.
Previous studies have shown that surround inhibition can contribute to velocity tuning in visual cortex (Goodwin and Henry 1978; Patel and Sillito 1978). Masking inhibitory areas of the RF resulted in increased responses to rapidly moving stimuli, suggesting that inhibition was specifically involved with suppressing responses to rapidly moving stimuli (Goodwin and Henry 1978). Duration selectivity can also underlie selectivity for slowly moving stimuli (Duysens et al. 1985a,b). If a neuron does not respond to a stationary flashed stimulus of short duration, it is unlikely to respond to rapidly moving stimuli (Orban et al. 1985a,b), and preference for sustained stimuli can result in apparent tuning to slowly moving stimuli that remain in the RF for a sufficient time. The main goal of the present study was to determine whether and how surround inhibition contributes to velocity selectivity in the SC.
Single-unit extracellular recordings of neurons in superficial SC in anesthetized hamsters were made with and without occlusion of the nasal or temporal flanks of the inhibitory RF surround during presentation of a horizontally moving light stimulus. We found that masking the surround altered velocity tuning in most SC neurons. Surround inhibition was absent in the remaining neurons, and these instead exhibited duration sensitivity. Taken together with our previous findings (Huang and Pallas 2001; Razak et al. 2003), these results suggest that velocity tuning in the SC depends more on inhibitory circuitry than on the degree of feed-forward afferent convergence. An understanding of the contribution of inhibition to velocity tuning in the hamster SC, a model system well suited for developmental studies, will facilitate studies of the mechanisms underlying recovery from early brain damage or manipulations of sensory experience.
METHODS
Animals
Forty-three Syrian hamsters (Mesocricetus auratus) were used in this study. Experimental animals were bred in the Georgia State University (GSU) animal facility from breeding stock purchased from Charles River Laboratories (Wilmington, MA). All procedures used on animals were reviewed and approved by the GSU Animal Care and Use Committee and were consistent with National Institutes of Health and American Physiological Society guidelines.
Surgical procedures
Electrophysiological recordings were obtained from adult hamsters (≥3 mo of age) after anesthetization with urethane (0.7 g/ml, 0.03 ml/kg administered intraperitoneally in three to four aliquots spaced at 20-min intervals). Pupils were dilated and accommodation was paralyzed with a 10% ophthalmic atropine solution. Respiration rates and withdrawal reflexes were monitored to ensure a deep level of anesthesia appropriate for surgery, with supplemental doses of urethane given as warranted. After performing a craniotomy over the SC, the visual cortex was aspirated to facilitate viewing the surface of the SC for electrode placement. In all experiments reported here, compensatory responses in the SC were highly unlikely because visual cortex was ablated acutely. We contend that the decorticate preparation does not affect the interpretation of this study for four reasons. First, in hamsters, Rhoades and Chalupa (1978a) showed that only 31% of SC neurons were influenced by ipsilateral cortical stimulation. Second, in rodents, the influence of descending cortical connections on SC response properties is minimal (Fortin et al. 1999; Rhoades and Chalupa 1978a). The removal of cortex has been reported in previous studies to affect only direction selectivity of SC neurons, and not velocity tuning (Rhoades and Chalupa 1978b). The same has been reported in cats (Wickelgren and Sterling 1969) and rabbits (Graham et al. 1982). Third, velocity tuning in decorticate hamster SC (Razak et al. 2003) is similar to that found in intact hamster SC (Chalupa and Rhoades 1977; Stein and Dixon 1979). Fourth, acute ablation of cortical input does not affect surround inhibition of SC neurons (Wickelgren and Sterling 1969). The decortication used in this and our previous studies thus will not influence the interpretation of our results with respect to velocity tuning in intact hamster SC. The decorticate SC preparation was, however, essential to compare normal velocity-tuning mechanisms with results from our previous developmental studies using chronic blockade of NMDA receptors or ablation of caudal SC (Pallas and Finlay 1989; Razak and Pallas 2003; Razak et al. 2003).
To maintain eye position during the recording session without paralysis, the conjunctivum was stabilized with 6 – 0 silk suture anchored to the nose clamp. The optic disk was replotted after every electrode pass to confirm that eye position remained stable, which in hamsters is typically not a concern (Pallas and Finlay 1989). The eye was covered with a fitted plano contact lens for protection during the recording session, and the brain was kept covered with sterile saline.
Visual stimulation and electrophysiological recording
All of the single units recorded in this study had their RF centered within ±15° of the optic disk and were isolated within 200 μm of the SC surface, consistent with previous studies (Huang and Pallas 2001; Razak et al. 2003). Tungsten microelectrodes (1–3 MΩ; FHC, Bowdoinham, ME) were lowered perpendicular to the surface of the SC to isolate visually responsive cells in the retino-recipient superficial gray layer of the right SC. The approximate location of the excitatory receptive field (eRF) and the preferred stimulus velocity were determined, and a 14-in. computer monitor was moved to the location of the eRF at a distance of 40 cm from the left eye. A Sergeant Pepper graphics board (Number Nine, Cambridge, MA) was used in conjunction with “STIM ” software (kindly provided by Kaare Christian) to generate visual stimuli. Data were acquired by CED 1401 hardware and processed by Spike2 software (Cambridge Electronic Design, Cambridge, UK).
Superior colliculus neurons of many species prefer small, slowly moving spots (Pallas and Finlay 1989; Razak et al. 2003; Rhoades and Chalupa 1978a; Stein and Dixon 1979; Tiao and Blakemore 1976) and respond poorly to gratings or rapidly moving stimuli (>45°/s). The eRF diameter of each neuron was determined by sweeping a single spot of light (1° diameter) from the top to the bottom of the computer monitor screen (Fig. 1A). Successive sweeps started 2° lateral to the previous sweep, allowing a determination of the nasotemporal extent of the eRF. The light spot was swept at either 5 or 30°/s velocity, depending on whether the neuron responded better to slowly or rapidly moving stimuli. The estimated RF size did not change with the velocity of the stimulus used.
FIG. 1.
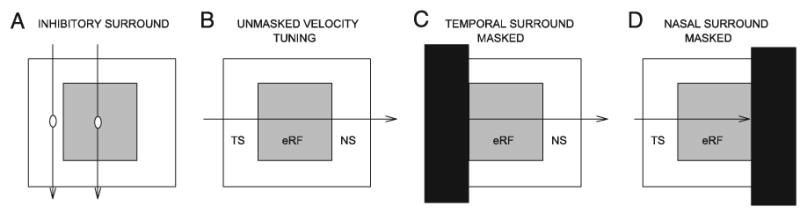
Schematic of the inhibitory surround masking protocol. A: horizontal extent of the excitatory receptive field (eRF) was determined by sweeping a 1° spot of light vertically from the top to the bottom of the monitor screen at horizontal interstimulus distances of 2°. Horizontal extent and strength of the inhibitory surround were determined by simultaneously sweeping two small (1°) spots of light vertically. While one spot moved through the center of the eRF at every sweep, the position of the second spot was incremented in steps of 2.6° to either side of the eRF center. Strength of surround inhibition was defined as the amount of reduction in the response to the center spot resulting from the presence of the second spot. B: velocity tuning was determined by sweeping a spot of light horizontally in a temporal-to-nasal direction through the inhibitory and the excitatory RF. Terms temporal surround (TS) and nasal surround (NS) are used to describe the surround locations relative to the nose of the animal. TS refers to surround locations on the side of the RF distal to the nose, whereas NS refers to the surround locations on the side of the RF proximal to the nose. In the unmasked condition, the hamster viewed the spot of light moving through the entire horizontal extent of the monitor. C and D: in the masking conditions, stimulus movement through either the TS or the NS was obscured from the animal’s view using an opaque barrier.
To determine the extent of the inhibitory surround, two spots of light (each 1° in diameter) were swept from the top to the bottom of the monitor screen (Fig. 1A). During each successive sweep, the second spot of light started 2.6° further away from the previous location, whereas the first spot was swept simultaneously through the center of the eRF. This allowed us to determine the spatial extent and strength of inhibition of the response to the first spot as caused by the second spot. The time interval between each sweep was set at 10 s to avoid adaptation. Each stimulus pair was repeated three to seven times. Responses in SC are highly reliable and vary little over time, in contrast to cortical responses. We chose not to use a light spot with progressively increasing diameter to determine surround inhibition (as in e.g. Binns and Salt 1997) because most SC neurons are selective for stimulus size and will decrease their response to a larger stimulus even if it lies entirely within their excitatory RF (Pallas and Finlay 1989; Razak et al. 2003; Stein and Dixon 1979).
Determination of velocity selectivity
Neural selectivity for stimulus velocity was determined by sweeping a 2.5° diameter spot of light at 5 to 45°/s increasing at 5°/s intervals. For most of the experiments in this study, the direction of stimulus sweep was temporal to nasal (Fig. 1B). In a few cases we also measured responses to nasotemporally swept stimuli. The choice of stimulus velocities used was guided by previous results showing that the majority of hamster SC neurons are selective for slowly moving (≤ 10°/s) stimuli (Chalupa and Rhoades 1977; Pallas and Finlay 1989; Razak et al. 2003; Stein and Dixon 1979; Tiao and Blakemore 1976). Each stimulus set was usually repeated at least five times, although fewer trials were collected in some cases where a large number of tests were being done on the neuron. In all cases, the responses were quite consistent, as reflected in the small error bars when between trial comparisons were made.
Neurons were categorized as low-pass (LP), band-pass (BP), high-pass (HP), or nonselective (NS) (Razak et al. 2003). A neuron was defined as low-pass if its response to a stimulus moving between 5 and 15°/s was at least twice that of the least-preferred stimulus. High-pass neurons were those that responded best to stimuli moving faster than 25°/s, and that responded at < 50% of their maximum to the lowest velocity tested, 5°/s. Neurons in the band-pass category responded best to an intermediate velocity, with responses falling below 50% of their maximum at the lowest and the highest velocities tested. Low-pass neurons generally did not respond better to stationary stimuli but the response of high-pass neurons would be expected to decline with stimuli faster than 45°/s (Chalupa and Rhoades 1977). Nonselective neurons by definition responded at > 50% of maximum at all velocities tested.
Determination of mechanisms of velocity tuning
To determine whether suppression from the inhibitory surround contributes to stimulus velocity tuning in the hamster retinocollicular pathway, an opaque barrier was used to mask various parts of the surround (Fig. 1, C and D). In this study, a masking paradigm was used instead of local pharmacological manipulation of γ-aminobutyric acid (GABA) receptors because we were specifically interested in analyzing the contribution of surround inhibition, and not within-field inhibition, to velocity tuning. The terms temporal surround (TS) and nasal surround (NS) are used to describe the surround locations relative to the nose of the animal. TS refers to surround locations on the side of the RF distal to the nose, whereas NS refers to the surround locations on the side of the RF proximal to the nose. The mask was positioned to cover the TS or NS up to the edge of the eRF. Velocity tuning was then determined as above with either the NS or the TS masked. Velocity tuning was determined again after removal of the masks to ensure that the effects of masking were temporary and attributable to the masks alone.
Determination of stimulus duration selectivity
After determination of the role of surround inhibition in each neuron’s velocity-tuning profile, its selectivity for stimulus duration was determined. A stationary light spot of 2.5° diameter was flashed for different durations in the center of the eRF. Almost all SC neurons in the hamster respond to both the onset and the offset of flashed stimuli. In this study, only the stimulus ON duration was manipulated. Therefore the ON response of the neuron to 15 successive flashes was averaged for analysis of flash duration selectivity. In most cases four durations (1 s and 500, 200, and 100 ms) were tested. In a few neurons an additional duration (50 ms) was also tested. During the initial stages of this study, a 2-s interstimulus interval was used for the 1-s stimulus duration and a 1-s interstimulus interval was used for the shorter stimulus durations. We were concerned that this difference in interstimulus interval could cause differences in neural adaptation, and thus the interstimulus interval was changed to 2 s for all stimulus durations. Data using both presentation rates were combined because no significant differences were observed in the results. The time interval between the first and last action potentials evoked by the onset of a 1-s stationary stimulus was used as a measure of response duration.
Data analysis
We chose to use spike counts as a measure of neuronal selectivity for velocity. Velocity selectivity of neurons can also be measured using spike rate (cf. Movshon 1975). In the time-independent spike count method that we used, all of the spikes evoked by stimulus movement through the RF were counted regardless of the time spent by the stimulus within the RF (see Barlow et al. 1964). In the spike rate, space-independent method, the peak firing frequency is measured (Waleszczyk et al. 1999). The peak firing typically occurs while the stimulus traverses the center of the RF. However, our initial experiments suggested that the contribution of inhibition to velocity tuning was not flat across the RF, and the effect of masking was to enhance the response nearer to the edges of the RF, not at RF locations with maximal firing. In several neurons, when surround inhibition was masked, the response to a moving stimulus was initiated earlier than when the mask was absent. Moreover, the location of response enhancement was dependent on whether the neuron was low-pass, band-pass, or high-pass tuned. Because of the lack of spontaneous activity in superficial SC neurons in anesthetized adult hamsters (Huang and Pallas 2001), and the long interstimulus intervals used in this study, it was possible to measure accurately the number of stimulus-evoked responses at each velocity without recourse to subtraction of background activity. With the space-independent method, if we had used spike rate at the center of the RF as a metric, the role of inhibition in velocity tuning would have been missed. One potential issue with using the time-independent method is the effect of duration of a stimulus within the RF on response level. The flash-duration–sensitivity data, however, allowed us to distinguish between velocity tuning that arises from inhibition and the apparent low-velocity tuning that arises from duration selectivity. Moreover, the distribution of preferred velocities in our studies (Huang and Pallas 2001; Pallas and Finlay 1989; Razak et al. 2003; this study) agrees closely with that of others using a spike rate measurement method in the hamster SC (Chalupa and Rhoades 1977; Stein and Dixon 1979), suggesting that spike count and spike rate measures produce similar results. We also estimated velocity tuning using peak firing rate in 51 neurons recorded. Neurons that were not duration selective showed similar velocity-tuning profiles regardless of whether spike count or peak firing rate estimates were used. For example, the neuron shown in Fig. 2, A and B responded weakly to rapidly moving stimuli. This neuron’s velocity-tuning profile was similar regardless of whether peak rate (Fig. 2A) or spike count (Fig. 2B) was used to measure response magnitude. The neuron shown in Fig. 2, C and D preferred rapidly moving stimuli and its tuning profile was similar for both methods. Many duration-selective neurons did show different velocity selectivity for different methods of counting. Spike count estimates may not accurately reflect velocity tuning in these neurons. Therefore the mechanisms of velocity tuning are discussed only for neurons that were nonselective for stimulus duration.
FIG. 2.
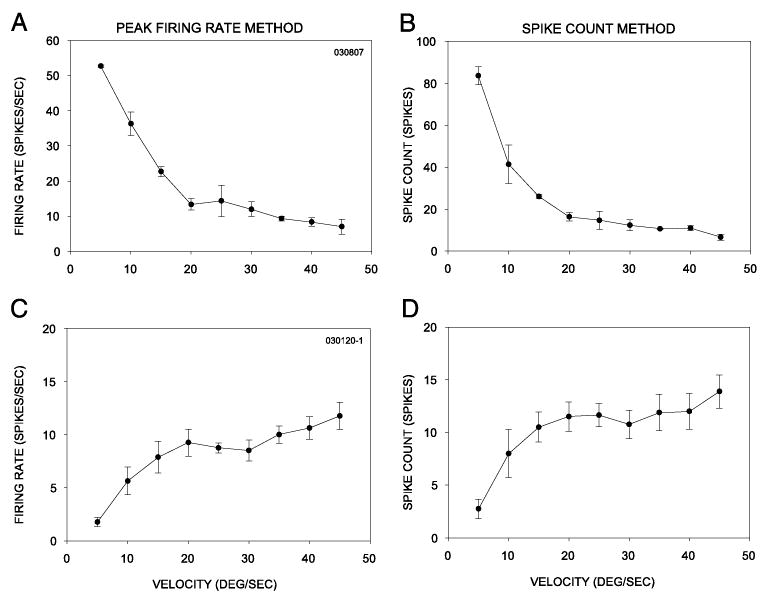
Comparison of velocity selectivity determined using the peak firing rate (A, C) method and the spike count (B, D) method. See text for details.
To determine whether physical masking of the inhibitory surround had any effect on velocity tuning in the SC, responses at each velocity in the control and masked conditions were statistically compared using a two-way ANOVA, with a Tukey test for post hoc, pairwise comparisons. For comparison of properties across the recorded population of neurons, regardless of the response magnitude in any particular neuron, normalized curves were constructed by dividing the response at each velocity by the response to the preferred velocity. To determine the temporal characteristics of the responses to flashed stimuli, both peristimulus time histograms (PSTHs) and raster plots were constructed from the raw data using the Spike2 software. A t-test was used to determine whether differences existed in the mean response durations for the 1-s stimulus flashes across the various categories of velocity tuning. In all of the figures, variability of responses across stimulus repetitions is depicted as ± SE.
RESULTS
We present results from single-unit extracellular recordings of 66 neurons from the superficial gray layer of the SC assessed for velocity sensitivity and surround inhibition (Table 1). With a stimulus passing from temporal to nasal locations across the center of the RF, 64 of the 66 neurons were found to respond differentially to different stimulus velocities in the range of 5 to 45°/s. The majority of the neurons (38/64) responded best to slowly moving stimuli (defined as low-pass neurons), 14 responded best to rapidly moving stimuli (high pass), and 12 responded best to intermediate velocities (band-pass). This distribution of velocity sensitivity across the population of SC neurons is similar to that found previously in hamsters (Chalupa and Rhoades 1977; Huang and Pallas 2001; Pallas and Finlay 1989; Tiao and Blakemore 1976) and in frogs and fish (for review see Ewert 1974; Vanegas 1984).
TABLE 1.
Distribution of neuron classifications
| Symmetric Surround | Asymmetric Surround | Weak Surround | |
|---|---|---|---|
| Low-pass | 0 | 17 | 21 |
| High-pass | 14 | 0 | 0 |
| Band-pass | 12 | 0 | 0 |
| Nonselective | 0 | 0 | 2 |
Each value represents the number of neurons (from a total of 66) with the indicated combinations of surround inhibition and velocity tuning profiles. Low/high/band-pass and nonselective are the designations for the velocity tuning profiles, whereas symmetric, asymmetric, and absent describe the surround inhibition characteristics.
The detailed geometry of the suppressive surround was determined in each of the 66 neurons using dual stimuli. One stimulus was swept across the center of the excitatory receptive field (eRF) as a second, simultaneous stimulus was swept at progressively greater distances from the first at 2.6° intervals (Fig. 1; see METHODS). In 43 of the 66 neurons assessed (65%), surround inhibition was found to be present and declined progressively as the second stimulus was swept at increasing distances from the edge of the eRF. Surround inhibition in this population was either spatially symmetric (26/43 neurons) or asymmetric (17/43 neurons). In the 17 neurons with an asymmetric surround, mapping with the dual stimuli revealed asymmetries in the strength of surround inhibition between the nasal (NS) and temporal (TS) surround. All were more strongly inhibited by stimuli in the NS than in the TS (Fig. 3, A, C, and E) (two-way ANOVA, Tukey test for post hoc, pairwise comparisons of similar locations on either side of the eRF, *P < 0.05). In the other 23/66 (35%) neurons, little to no surround suppression was revealed by the dual-stimulation procedure. These results suggested that two populations of SC neurons exist, one with a suppressive surround and one without.
FIG. 3.
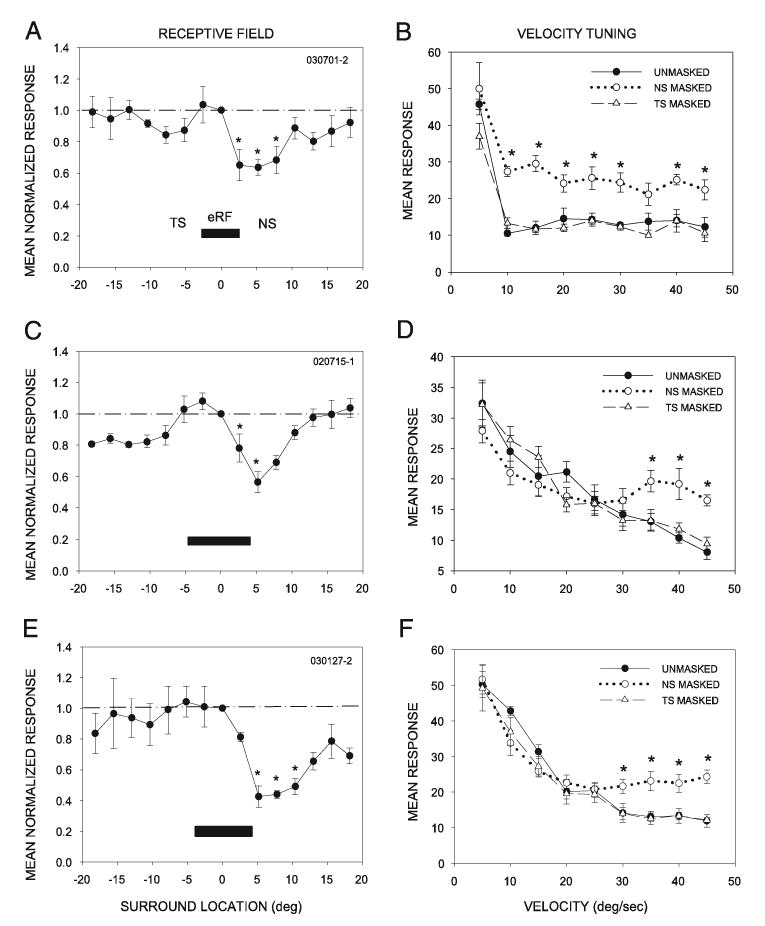
Asymmetric inhibition from the NS contributes to low-pass velocity tuning. Data from 3 representative neurons are shown. A: spatial structure of the receptive field in a single, representative low-pass velocity tuned neuron. Horizontal extent of the eRF, determined as described in Fig. 1, is depicted by the black rectangle. Surround inhibition was measured by comparing the response to a single spot swept through the center of the eRF to the response to 2 spots of light (see METHODS and Fig. 1). y-axis shows the response to both spots of light as normalized to the response to the central spot (dashed line); x-axis shows the distance of the second spot from the first in the horizontal plane, with 0° as the center of the eRF, positive values nasal and negative values temporal in location. This neuron had an eRF diameter of 6° and an asymmetrically shaped inhibitory surround. Asterisks indicate surround locations where inhibition within the NS was significantly stronger than that within the TS (P < 0.05). B: an opaque barrier was used to occlude either the TS or the NS to determine the contribution of that portion of the inhibitory surround to velocity tuning. Masking the NS enhanced the neural response to all velocities except the preferred velocity (5°/s) (*P < 0.05). Masking the TS had no significant effect on velocity tuning (P < 0.05 at all velocities). C: this neuron had an eRF of 8° and an asymmetric inhibitory surround. D: masking the NS caused the neuron to respond better to more rapidly moving stimuli compared with the unmasked condition (*P < 0.05). Masking the TS had no significant effect on velocity tuning (P > 0.05 at all velocities). E and F: another example of a low-pass velocity-tuned neuron with a strongly asymmetric inhibitory surround and a loss of velocity tuning after masking of the NS, but not the TS.
Spatially asymmetric surround inhibition contributes to low-pass velocity tuning
The contribution of surround inhibition to the velocity selectivity of each neuron was determined by recording the response to a single stimulus moving at varying velocities in the temporonasal direction, with and without masking the nasal or temporal flank of the receptive field surround (Fig. 1). We found that all 17 neurons with spatially asymmetric surround inhibition were selective for slowly moving stimuli (LP selective, e.g., Fig. 3, B, D, and F, unmasked). Surround inhibition clearly contributed to the velocity tuning in these neurons. Masking the nasal flank of the RF (NS), thus causing the temporonasally directed stimulus to disappear at the far edge of the eRF, resulted in an improved response of the neurons to higher-velocity stimuli, thus reducing their selectivity for low-velocity stimuli (Fig. 3, B, D, and F, NS masked). We refer to these neurons as low-pass/inhibition (LP/I) neurons. In 82.3% of these LP/I neurons (14/17), only NS masking and not TS masking affected velocity tuning (two-way ANOVA, Tukey test for post hoc, pairwise comparison at velocities between 10 and 45°/s). The responses with TS masking were similar to those in the unmasked condition for these 14 neurons (Fig. 3, B, D, and F, TS masked). Masking the NS or TS had no effect when stimuli were moved in the nasotemporal direction (n = 3). The NS masking–induced increase in response level at higher stimulus velocities argues that inhibition from the NS contributed to velocity tuning in the class of neurons represented in Fig. 3 by suppressing responses to more rapidly moving stimuli. Their responses to slowly moving stimuli were still considerably higher than their responses to rapidly moving stimuli, however, suggesting that asymmetric inhibition plays an important, but not exclusive, role in shaping low-pass velocity tuning in SC neurons.
LP/I neurons respond phasically and are not selective for stimulus duration
In 54 of the 66 neurons, stimulus-duration selectivity was also measured to explore the possibility that sensitivity to slowly moving stimuli could result from a preference to long-duration stimuli rather than movement (a slowly moving stimulus spends more time in the eRF than a rapidly moving stimulus). To assess duration sensitivity, the response to a stationary stimulus of varying duration was measured. The responses of most LP/I neurons (13/17) were independent of the duration of a stationary flash stimulus (e.g., Fig. 4, A–C). These results are consistent with the contention that velocity tuning in the LP/I neurons results primarily from asymmetries in surround suppression. Insensitivity to stimulus duration might be expected to result from a failure to respond tonically to prolonged stimulation. Indeed, we found that LP/I neurons responded phasically at stimulus onset. In raster and PSTH plots from representative LP/I neurons (Fig. 4, D–F), the onset response to stationary stimuli was dominated by the phasic component, with no additional response until the offset of the stimulus. Taken together, these results suggest that the absence of tonic responses in the continued presence of a stimulus underlies the poor stimulus-duration sensitivity of these neurons, and that when surround inhibition is masked, they are capable of responding well to rapidly moving stimuli. These results provide further evidence that surround inhibition shapes low-pass velocity tuning by suppressing excitation that otherwise would be elicited by rapidly moving stimuli.
FIG. 4.
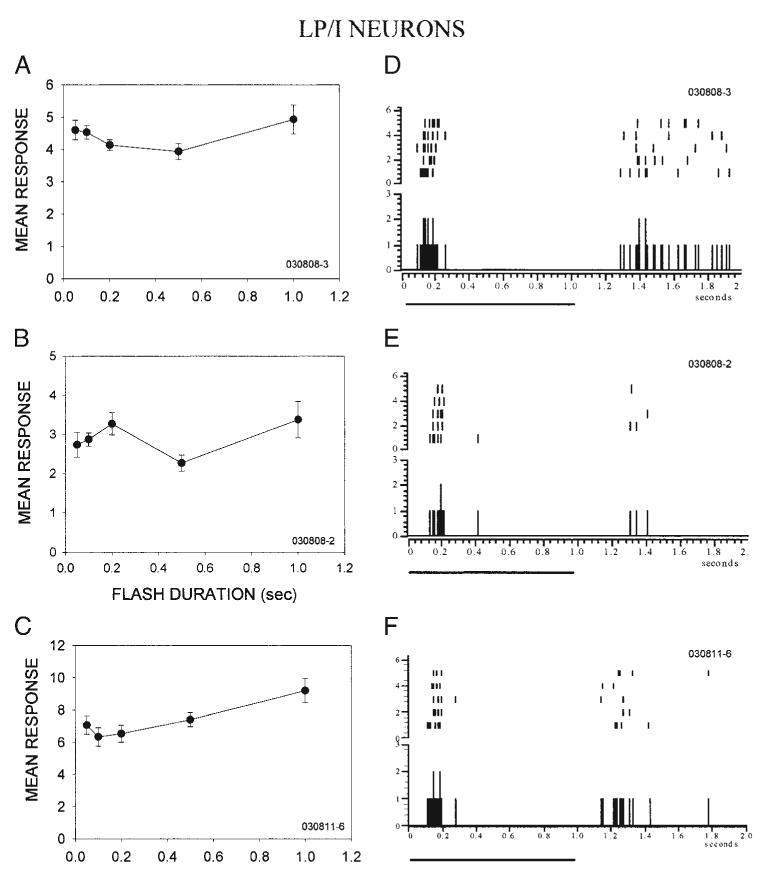
Low-pass neurons that depend on inhibition for velocity tuning are poorly tuned for stimulus duration and respond phasically to stimulus onset. A–C: 3 representative low-pass/inhibition (LP/I) neurons responded similarly regardless of the duration of a stationary, flashed visual stimulus. D and E: peri-stimulus time histograms (PSTHs) and raster plots of responses to the onset and offset of a 1-s flash in the same 3 neurons as in A–C. Five repetitions are shown. Both neurons responded phasically to stimulus onset. Black line below the PSTH denotes stimulus duration.
Surround inhibition is absent in some SC neurons
Surround inhibition was absent in 23/66 SC neurons. If surround inhibition is necessary for velocity sensitivity, then this group of neurons should be insensitive to velocity, although this was not the case. As shown with the two representative neurons in Fig. 5, no detectable surround inhibition was present (Fig. 5, A and C). Placing a mask just outside of the eRF did not alter the overall response to stimuli moving at different velocities, suggesting that delayed or advanced inhibition was not present in the surround of these neurons (Fig. 5, B and D). Nonetheless 21 of 23 neurons in this population of neurons exhibited LP velocity sensitivity. It is possible that inhibition in the surround cannot be produced in this neuron type by the simultaneous center and surround stimulation used here. Changing the temporal intervals between presentations of the two spots might reveal inhibition (Dawis et al. 1984).
FIG. 5.
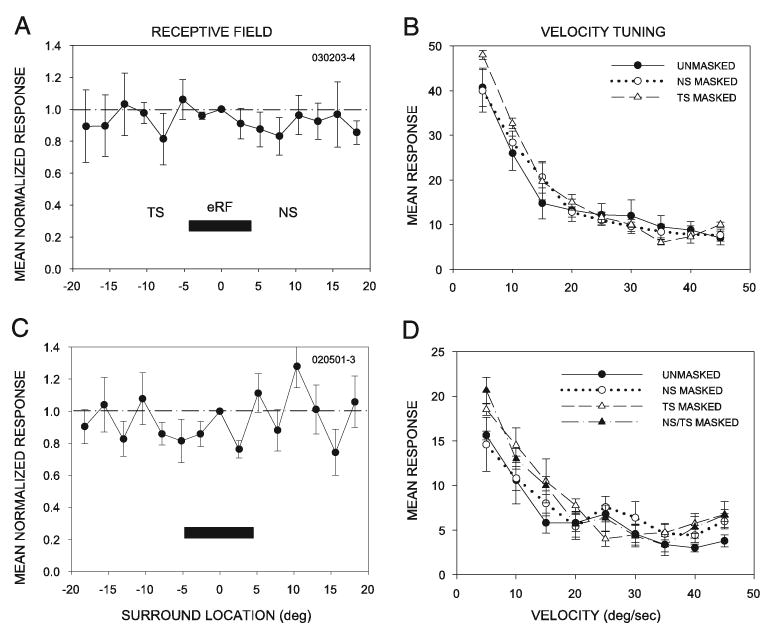
In neurons with no inhibitory surround, velocity tuning was unaffected by masking. A: neuron with no detectable surround inhibition. Response of this neuron to a second spot of light outside the eRF was not different from the response to a single spot of light. B: placing the opaque barrier at the edge of the excitatory RF on either the NS or the TS had no significant effect on velocity tuning in the neuron shown in A. C: in a second representative example, again no detectable inhibition was present outside the eRF. D: placing the mask at the nasal, the temporal, or on both sides of the eRF had no significant effect on its velocity tuning.
Neurons without surround inhibition exhibit stimulus-duration selectivity and longer-duration responses
Neurons without surround inhibition were sensitive to the duration of stationary, flashed stimuli (Fig. 6), and are thus referred to as duration selective (DS). In contrast to the LP/I neurons, responses of the majority of DS neurons (18/23) to the shortest duration stimuli were significantly lower (P < 0.05) than at the longest-duration stimuli tested (1 s) (Fig. 6, A–C). This dependency of response magnitude on stimulus duration could produce an apparent selectivity for slowly moving stimuli, as observed in 21/23 of the neurons without surround inhibition. These neurons might thus be described as stimulus-duration coders. Although surround inhibition clearly does not play a role in velocity tuning in these neurons, it is not clear from the methods used here whether they exhibit true velocity tuning as well as duration selectivity. Neurons would be designated duration selective if their response duration, in addition to their total response magnitude, depended on stimulus duration. An important corollary is that these neurons should have relatively tonic rather than phasic responses. PSTHs and raster plots of responses to 1-s-duration, flashed stimuli reveal that the responses of duration selective (DS) neurons are relatively tonic and longer lasting than in the LP/I neurons (Fig. 6, D–F). The representative neurons shown in Fig. 6 responded strongly to the onset of the flash, and continued to respond for ≥ 600 ms after stimulus onset. This response pattern causes them to respond better to slowly moving stimuli than to rapidly moving stimuli.
FIG. 6.
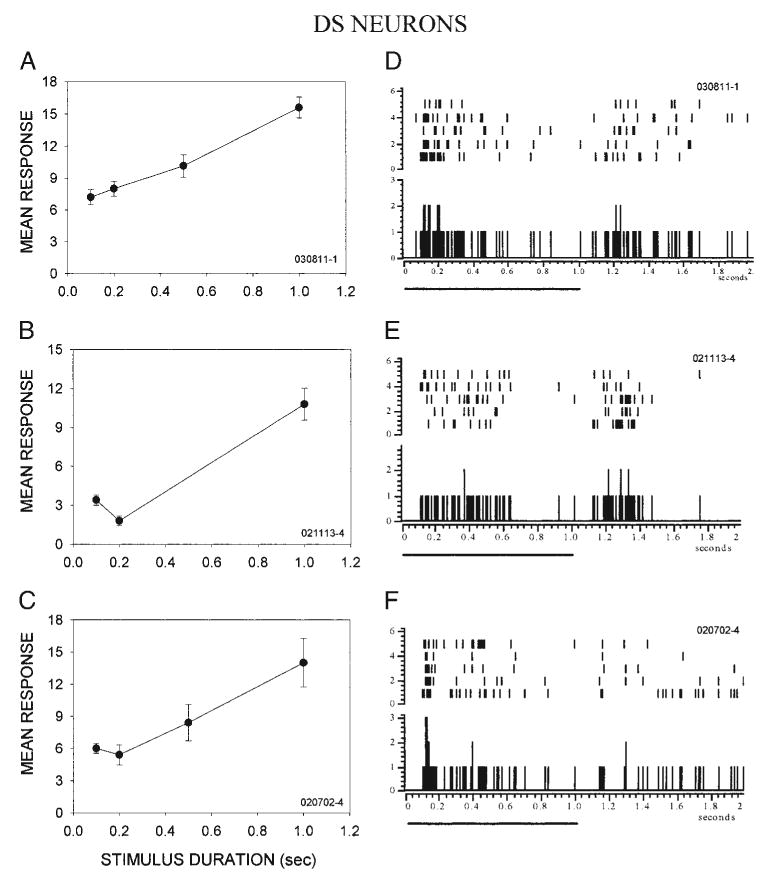
Low-pass neurons with weak surround inhibition are strongly selective for stimulus duration and exhibit relatively sustained firing. A–C: examples of duration selectivity in 3 superior colliculus (SC) neurons. As the stimulus duration was increased from 50 ms to 1 s, the onset response of these duration-sensitive (DS) neurons became stronger. In each neuron, the response to the shortest stimulus duration was < 50% of the maximal response. D–F: discharge patterns in the same DS neurons shown in A–C. Unlike LP/I neurons, the response of DS neurons to stationary stimuli was relatively sustained. Solid line below each PSTH indicates the duration of the stimulus.
Taken together, these data show that neurons with surround inhibition tend to have phasic discharges to a flashed stimulus and poor duration selectivity. These neurons respond best to slow stimuli because their responsiveness to fast stimuli is reduced by inhibition from the surround. Conversely, neurons without surround inhibition tend to have tonic discharges to flashed stimuli and strongly prefer longer duration stimuli. Because of this they are unable to respond to rapidly moving stimuli that occupy the RF for only a short time.
The pooled data in Fig. 7, show that in LP/I neurons, the surround inhibition was significantly stronger in the NS than in the TS (Fig. 7A). In the neurons that were unaffected by masking, surround inhibition was weak or absent. Significant reductions in velocity selectivity were observed when the nasal but not the temporal surround was masked (Fig. 7B). This asymmetry in the masking effect suggests that inhibition arising after the stimulus crosses the eRF suppresses responses to rapidly moving stimuli in a backward-masking fashion. Neurons without significant surround inhibition were strongly selective for stimulus duration as a population (Fig. 7C). In contrast, the LP/I neurons showed little to no sensitivity for duration. A comparison between the two types of neurons (LP/I and duration-sensitive groups) showed that normalized response magnitude at the shorter stimulus durations (100 and 200 ms) was significantly different (two-way ANOVA, Tukey test for all post hoc pairwise comparisons, *P < 0.05). The duration of neural responses to stimulus onset correlated with the presence or absence of surround inhibition, and thus the mechanism underlying differences in responsiveness to rapidly versus slowly moving stimuli. The ON response of DS neurons to 1-s flashed stimuli was significantly longer in DS than in LP/I neurons (mean ± SE: DS, 349.2 ± 26.26 ms; LP/I, 105.4 ± 12.97 ms, P < 0.001; distribution of response durations shown in Fig. 7D). Thus as a population, LP/I and DS neurons differed not only in their duration selectivity, but also in whether surround inhibition was present and in their response duration, suggesting that they belong to different neuronal classes.
Fig. 7.
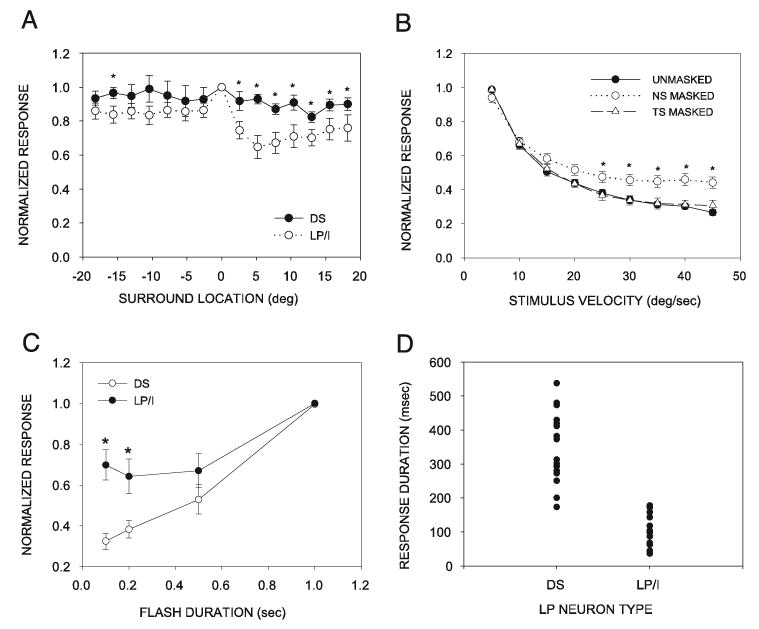
Summary of the contribution of surround inhibition and duration selectivity to low-pass velocity tuning in the SC. A: pooled data comparing the population of LP/I neurons to the population of duration-selective (DS) neurons show that their masking-induced changes in surround inhibition (*P < 0.05) exist primarily in the nasal surround. Difference in the strength of inhibition between the 2 LP classes at similar surround locations was significant at all NS locations (positive x-axis numbers). Difference was significant at only one TS location (negative x-axis numbers). B: when DS and LP/I neuron data are pooled, it is still the case that velocity tuning of the entire population of LP neurons was significantly affected (*P < 0.05) when the NS was masked, but not when the TS was masked. Significant effects of masking were observed at the 5 highest velocities used. C: stimulus duration selectivity curve of each neuron was normalized by the maximum response (usually the response to a 1-s flash). Mean (±SE) normalized stimulus duration selectivity curves of neurons with different velocity preferences show that the DS neuron category was most strongly selective for stimulus duration. Stimulus duration selectivity of LP/I neurons was weaker than that of DS neurons, primarily because of higher relative responses to the 100- and 200-ms stimuli (*P < 0.05). D: distribution of response duration after the onset of a 1-s stimulus duration, measured as the time interval between the first and the last action potential, was different between DS and LP/I neurons.
If asymmetries in surround inhibition contribute to velocity tuning in LP/I neurons, then selectivity should be higher for stimuli moving in the temporonasal direction than in the nasotemporal direction. No such difference should exist for DS neurons. We were able to completely assess stimulus velocity tuning in both stimulus sweep directions in nine DS and four LP/I neurons (Fig. 8). As predicted, velocity tuning in DS neurons was similar for both movement directions (Fig. 8A). In contrast, in the LP/I neurons, the response to rapidly moving stimuli was more effectively suppressed when the stimulus moved in the temporonasal direction than in the opposite direction (Fig. 8B). The difference reached statistical significance for the two highest velocities, even in this small sample.
Fig. 8.
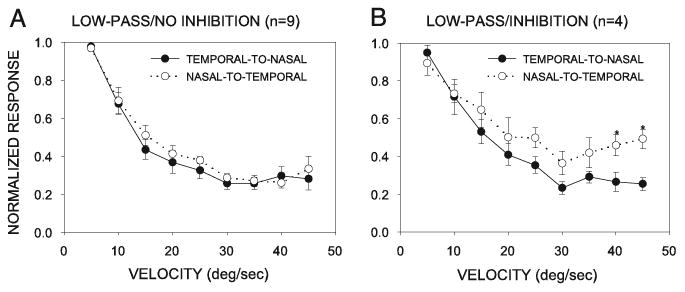
Direction-dependent differences in velocity tuning. Velocity selectivity in the nasal-to-temporal direction of stimulus movement was lower than that in the temporal-to-nasal direction of movement in the LP/I neurons (B), but not DS neurons (A). Data were normalized to the highest response level.
Spatially symmetric surround inhibition underlies velocity tuning in high-pass and band-pass velocity-tuned neurons
Neurons selective for slowly moving stimuli were in the majority in the superficial gray layer of the SC, but we were able to isolate a substantial number of neurons that preferred more rapidly moving stimuli. All of the neurons with high-pass and band-pass velocity tuning exhibited surround inhibition to some degree. In the two HP neuron examples shown (Fig. 9, A and C), the response to a center spot of light was reduced by ≥ 30% when a second spot of light was swept within either the TS or the NS. Unlike the LP/I neurons, HP and BP neurons had inhibition on both sides of the eRF when tested with the dual-stimulus configuration (Fig. 1A).
Fig. 9.
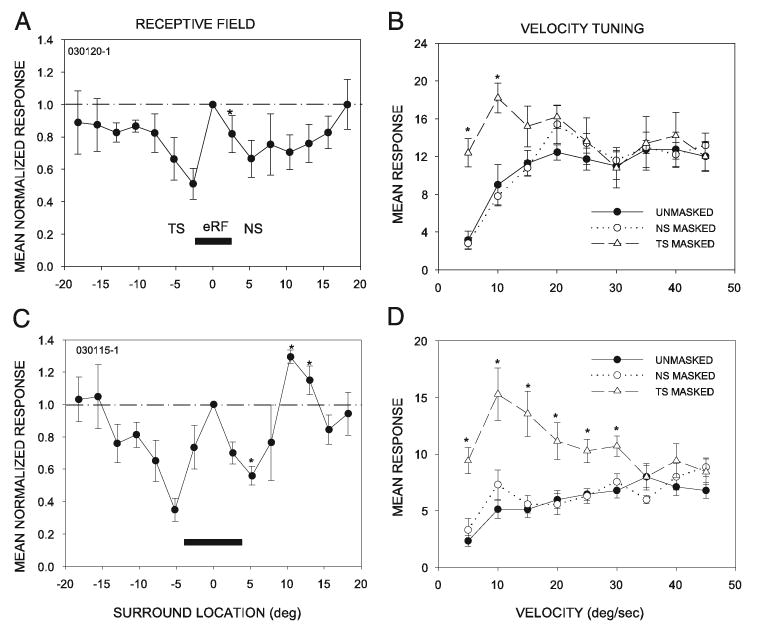
Masking the temporal surround decreases selectivity of high-pass velocity tuned neurons. Two examples of high-pass neurons, illustrating their receptive field structure (A, C) and the effects of masking (B, D) on velocity tuning. In high-pass neurons, inhibition was present on both sides of the eRF. In contrast to low-pass neurons, masking the NS had no effect on velocity tuning. Masking the TS, however, revealed responses to low-velocity stimuli. Asterisks in A and C show locations where the amount of suppression was significantly different on one side of the RF center than on the other, as in Fig. 2. Asterisks in B and D show significant effects of masking the temporal surround (*P < 0.05).
Masking the inhibitory surround altered velocity tuning in 79% (11/14) of high-pass neurons and 83% (10/12) of bandpass neurons, suggesting that the surround is important in shaping velocity tuning in the majority of these neurons. In the unmasked condition, neurons responded poorly to velocities < 10°/s (Fig. 9, B and D). Masking revealed asymmetries in the organization of surround inhibition. When the TS was masked, the neurons were rendered completely nonselective to velocity, as a result of increased responses at lower, nonpreferred velocities (two-way ANOVA, Tukey test for post hoc pairwise comparisons at velocities between 5 and 10°/s in B and 5 to 30°/s in D, *P < 0.05). In contrast, masking the NS had no effect.
The effect of masking on velocity tuning in BP neurons was similar to that seen in HP neurons. In band-pass neurons, as in HP neurons, the responses to a moving spot of light were reduced by adding a second spot of light on either side of the eRF (Fig. 10, A and C). In the unmasked condition, the responses to a spot moving at 5 or > 30°/s velocity were low (Fig. 10, B and D). When the TS was masked the responses to slowly moving stimuli were significantly enhanced (two-way ANOVA, Tukey test for post hoc pairwise comparisons, *P < 0.05), giving these neurons a low-pass appearance under TS masking. The responses to rapidly moving stimuli were not significantly altered by TS masking. Masking the NS had no effect at any velocity in these neurons. Thus velocity selectivity in HP and BP neurons depended on inhibition arising before the stimulus enters the excitatory RF (forward masking) to reduce their responsiveness to slowly moving stimuli.
Fig. 10.
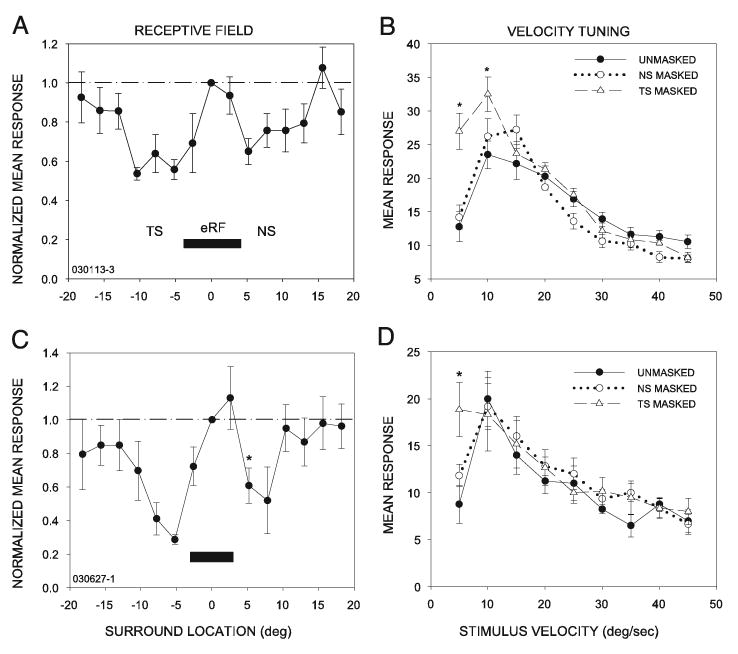
Masking the temporal surround decreases velocity selectivity of band-pass (BP) neurons. BP neurons were similar to high-pass neurons in that inhibition was present on both sides of the eRF (A, C), and masking the TS decreased tuning, revealing responses to slower stimuli (B, D). For the neuron shown in B, masking the TS increased responsiveness to stimuli moving at 5 and 10°/s (*P < 0.05) without a significant effect on neural response levels to higher velocity stimuli. For the neuron shown in D, a significant effect of masking the TS was seen only at 5°/s. In both of these neurons, masking the NS had no significant effect. Asterisks in A and C show locations where the amount of suppression was significantly different on one side of the RF center than on the other, as in Figs. 3 and 9.
High-pass neurons are not selective for stimulus duration
In high-pass neurons, inhibition from the surround suppresses the responses to slowly moving stimuli. In the absence of surround stimulation, HP neurons respond similarly to all stimulus velocities, suggesting that they have poor stimulus duration selectivity. This suggestion was supported; all HP (14/14) neurons were only weakly selective for stimulus duration. Two representative HP neurons are shown in Fig. 11. Increasing the stimulus duration from 100 ms to 1 s had no effect on their response magnitude (Fig. 11, A and C), demonstrating that the responsiveness of these HP neurons to stationary, flashed stimuli is not dependent on the length of time a stimulus spends inside the RF. The temporal response characteristics of HP neurons to stationary, flashed stimuli were similar to those of LP/I neurons. The response to flashes was dominated by a phasic component, with little or no sustained component (Fig. 11, B and D). An extreme example of this characteristic is shown in Fig. 11D. This HP neuron responded with a first spike latency of around 90 ms, and responded to both the onset and the offset of the flash. The response of this neuron typically consisted of only one or two precisely timed spikes at stimulus onset, followed by a longer response at offset. Thus surround inhibition reduces responsiveness to slowly moving stimuli, and strong responses to brief stimuli may explain the tuning to rapidly moving stimuli in HP neurons.
Fig. 11.
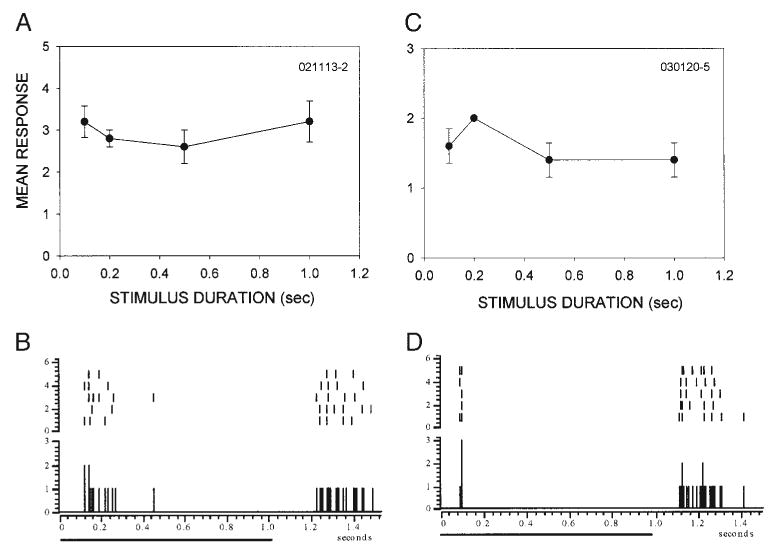
High-pass (HP) neurons are poorly selective for stimulus duration. A and C: stimulus duration tuning in 2 representative HP neurons. Both neurons were insensitive to the duration of stationary flashed stimuli. B and D: similarly to LP/I neurons, HP neurons displayed phasic responses to stimulus onset.
Band-pass neurons are selective for stimulus duration
In band-pass neurons, surround inhibition reduced the responsiveness to slowly moving stimuli, but masking the surround did not influence the response to rapidly moving stimuli. We next investigated the mechanisms underlying the reduced responsiveness of BP neurons to fast stimuli. We found that most band-pass neurons (9/12), like the DS neurons, were sensitive to the duration of stationary, flashed stimuli. This suggests that the reduced response to rapidly moving stimuli in BP neurons may have resulted from duration sensitivity. As a population, BP neurons varied more in their temporal response characteristics than did DS, LP/I, or HP neurons. The two examples shown in Fig. 12 represent the extremes in stimulus duration sensitivity among the BP neurons recorded. As stimulus velocity deviated from the preferred velocity, the response level declined abruptly for slower velocities and more gradually for higher velocities (Fig. 12, A and D). Although both neurons were sensitive to stimulus duration, their degree of selectivity differed (Fig. 12, B and E). Band-pass neurons also displayed a wide range of response patterns (Fig. 12, C and F). The neuron shown in Fig. 12C resembled DS neurons in its temporal response profile because of the tonic component to its response. The neuron shown in Fig. 12F, in contrast, responded phasically to stimulus onset and had a delayed, weaker, but consistent ON response. Despite the differences in stimulus duration selectivity and temporal response characteristics, neurons in this class exhibited similar velocity-tuning profiles, suggesting that a strong correlation may not exist between stimulus duration selectivity and the rate at which neuronal responses decrease with increasing velocity in BP neurons.
Fig. 12.
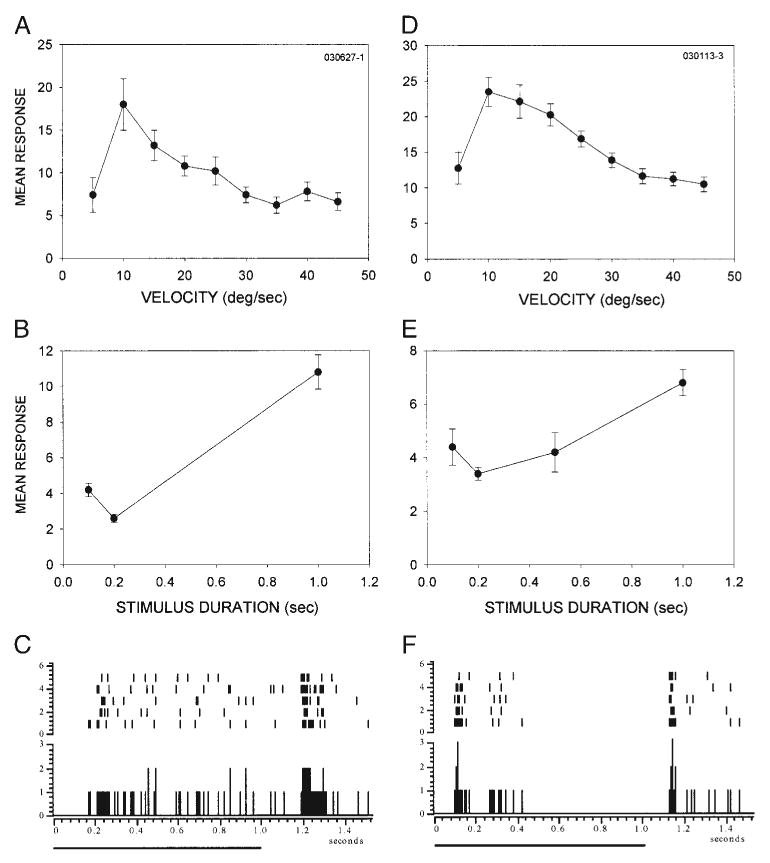
BP neurons are sensitive for stimulus duration. A and D: velocity tuning of two BP neurons with similar tuning characteristics. B and E: both neurons were sensitive to the duration of stationary stimuli, albeit with different selectivity. C and F: BP neurons displayed either a sustained response to stimulus onset (C) or a mix of a phasic onset and a weaker delayed response (F).
High-pass and band-pass neurons: population summary
In summary, in HP and BP neurons, strong inhibition was present on both sides of the eRF (Fig. 13, A and B). In the HP neurons, there was no difference in the strength of inhibition produced with stimulation at similar distances from the center of the RF on the temporal or nasal surround. In the BP neurons, the strength of inhibition produced by the second spot was significantly weaker at 2.5° nasal from the center compared with the 2.5° temporal location. Because these SC neurons have RF diameters >5° (Huang and Pallas 2001; Razak et al. 2003; this study), this asymmetry was present within the RF, not the surround. It is unlikely to have contributed to the asymmetric effects of masking on velocity tuning because neurons with this asymmetry and those without it responded similarly to masking. For a stimulus moving in a temporonasal direction, inhibition arising from the temporal, but not the nasal, surround contributed to high-pass and band-pass velocity tuning (Fig. 13, C and D). Masking the NS did not cause an increase in the response to nonpreferred velocities in HP or BP neurons.
Fig. 13.
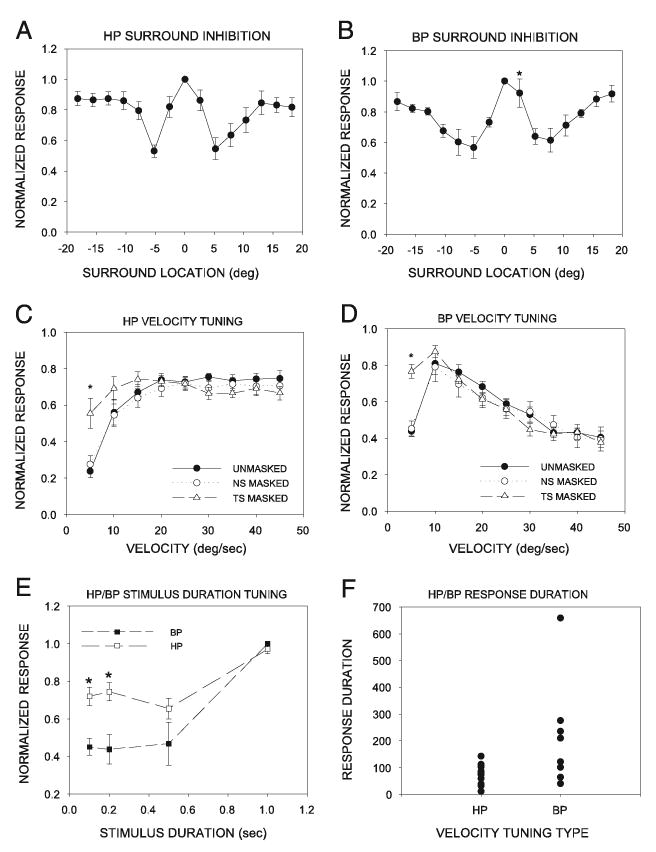
Summary of neural mechanisms of velocity tuning in HP and BP neurons. A: HP neurons exhibited surround inhibition on both sides of the eRF. B: like HP neurons, BP neurons also exhibited surround inhibition on both sides of the eRF. C: despite the presence of inhibition on both sides of the eRF, the velocity tuning of HP neurons was affected only when the TS was masked. These results suggest that in HP neurons, inhibition from the TS reduces responses to slowly moving stimuli. D: BP neurons showed enhanced responses to slowly moving stimuli only when the temporal surround was masked. As in the HP neurons, masking had no effect on the response of BP neurons to rapidly moving stimuli. E: HP neurons were poorly selective for stimulus duration. Mean (± SE) magnitudes of the responses at all stimulus durations were within 70% of each other. BP neurons were weakly selective to stimulus duration. Mean (± SE) response to short-duration stimuli decreased to < 50% of the maximum response level seen to long duration flashed stimuli. F: HP neurons responded phasically to a 1-s flash, whereas BP neurons exhibited a mixture of both long- and short-duration responses.
HP neurons were similar to LP/I neurons, in that they were only weakly selective for stimulus duration, and their response to stationary, flashed stimuli was dominated by the phasic component. The normalized mean response of the population of HP neurons to the two shortest stimulus durations tested was within 70% of the response to the 1-s flash (Fig. 13E). Response duration of HP neurons to the 1-s flash was as low as that seen in any other category of neurons (Fig. 13F).
Band-pass neurons were highly selective for stimulus duration and their mean response duration to 1-s flashed stimuli was significantly (mean ± SE: HP, 76.8 ±9.8 ms; BP, 193.7 ± 64.9 ms; P < 0.05) more sustained than that in HP neurons (Fig. 13, E and F). However, there was a higher degree of variability in the response patterns of BP neurons.
Nonselective neurons exhibit weak inhibitory surround and stimulus duration selectivity
Results from recordings of LP, HP, and BP neurons suggest that specific interactions with inhibition from the surround shape velocity tuning in the SC. The results thus predict that a lack of surround inhibition and poor stimulus duration selectivity would cause neurons to respond well at all velocities. This prediction was tested in the two neurons in our sample that could be classified as nonselective according to our criteria (see METHODS). Because velocity-insensitive neurons are rare in superficial SC, the sample size was necessarily small. Both of these neurons exhibited little inhibition in the surround and were insensitive to stimulus duration. The results from recordings of these two neurons support our contention that a lack of surround inhibition and poor stimulus duration selectivity should result in equal responsiveness to all stimulus velocities tested in this study.
Relationship between velocity-tuning profile, surround inhibition, and duration selectivity
A consistent observation in this study was that neurons with different velocity-tuning profiles exhibited characteristic combinations of surround inhibition and duration selectivity (Fig. 14). To quantify this relationship, we calculated indices of inhibition strength and duration selectivity. We represented the strength of surround inhibition with a surround inhibition index (SII), calculated as
Fig. 14.
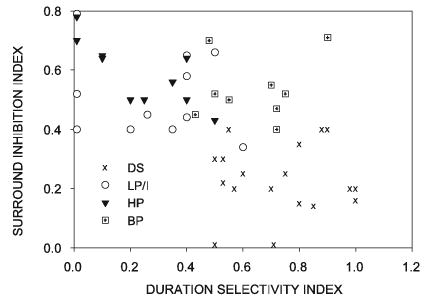
Neurons with different velocity tuning profiles differ in their surround inhibition and duration selectivity characteristics. Surround inhibition and duration sensitivity were quantified (see text). As defined here, the larger the duration selectivity index, the more selective the neuron is for flash duration; the larger the surround selectivity index, the more pronounced the inhibition. HP and LP/I neurons fell into a cluster characterized by low duration selectivity and strong surround inhibition. DS neurons had weak surround inhibition and high duration selectivity. BP neurons are duration tuned and show strong inhibition.
The SII can vary from 0 to 1, with 0 indicating no suppression and 1 indicating complete suppression of the response to a stimulus in the center of the RF by a second stimulus in the surround. Theoretically, SII could be negative if all surround locations showed facilitation, but we did not observe negative SII values in this study. The strength of duration selectivity is represented by the duration selectivity index (DSI), calculated as
The DSI can vary from 0 to 1, with 0 indicating that the neuron exhibits no selectivity for stimulus duration and 1 indicating strong duration selectivity. Although the DSI could be a negative number if the response to a 100-ms-duration flash is greater than that to the longer flash, we never observed this.
As seen in Fig. 14, neurons with different velocity-tuning profiles formed distinct clusters when their DSI was plotted against their SII. The LP/I and HP neurons exhibited strong surround inhibition and weak duration selectivity. The DS class of neurons exhibited little to no surround inhibition, but was strongly selective for stimulus duration. The BP neurons exhibited strong surround inhibition and duration selectivity. These results raise the possibility that the velocity-selective neurons constitute three distinct classes and are not continuously distributed with respect to surround inhibition and duration sensitivity, although anatomical description would be necessary to confirm whether this is the case.
DISCUSSION
In this study, we sought to determine the contribution of surround inhibition to velocity tuning of SC neurons in anesthetized, decorticate hamsters. We report that surround inhibition was present and contributed to velocity tuning in 65% of the neurons. All neurons with asymmetric surrounds were more strongly inhibited when the second stimulus was on the nasal side of the RF. The vast majority of these neurons were tuned to slowly moving stimuli (termed LP/I). Moreover, in LP/I neurons, spatial asymmetries in the inhibitory surround contributed to velocity tuning. In these neurons inhibition that arises after the stimulus exits the excitatory RF (backward masking) reduced the response to rapidly moving stimuli, creating low-pass velocity tuning.
In neurons that preferred high-velocity stimulus motion (HP), inhibition was present on both sides of the RF. In these neurons, responses to slowly moving stimuli were suppressed by inhibition arising before the stimulus entered the excitatory RF (forward masking). Both LP/I and HP neurons exhibited poor duration selectivity, whereas most neurons without surround inhibition were duration selective, suggesting a correlation between the presence of surround inhibition and duration selectivity. In neurons tuned to intermediate velocities (BP), the failure to respond to slowly moving stimuli was attributed to a mechanism similar to that seen in the HP neurons (forward masking). The failure of BP neurons to respond to rapidly moving stimuli may result from a requirement for longer-duration stimuli.
Comparison of surround inhibition and velocity tuning with other species
The two main response properties examined in this study were surround inhibition and velocity selectivity. Surround inhibition in SC neurons has been best studied in cats. Dreher and Hoffman (1973) showed that in the cat SC, close to 72% of the neurons showed at least one inhibitory flank. This proportion is similar to the 65% reported here for the hamster SC. Interestingly, 5.3% of the neurons exhibited asymmetric surrounds, but the functional relevance of asymmetric flanks was not clear. McIlwain and Buser (1968) reported that only 55% of cat SC neurons showed inhibitory surround. However, because they used spots of increasing diameter to determine inhibition, it is not clear whether the results reflect only surround inhibition, or whether they are confounded by the size selectivity of SC neurons. Both of these cat studies showed that inhibition became progressively weaker with distance from the RF center. Dreher and Hoffman (1973) reported that at distances > 15° from the center, inhibition is weak or absent. This is similar to observations in the hamster, in which surround inhibition is strong close to the edge of the excitatory RF and is weak for distances > 20° from the RF center. In rhesus monkeys, a larger percentage of neurons (82%) exhibited surround inhibition (Wallace et al. 1997). It remains unclear whether these are true species differences or are a result of the different stimulus and anesthesia paradigms used.
Rizzolatti et al. (1974) reported the presence of a second type of suppression in SC neurons of cats using a stimulus paradigm similar to the one used in this study. Here, inhibition was observed even when the second stimulus was 60° away from the excitatory stimulus. Moreover, there was no reduction in inhibitory strength with distance from the RF center. It was suggested that there might be two different inhibitory subregions: one, which is strongest near the excitatory RF, and another that is uniform in strength ≥60° from the center. We did not observe a similar diversity of inhibitory surrounds in the hamster SC.
Velocity selectivity in rodents appears to be biased toward slowly moving stimuli. In hamsters with intact cortex, Stein and Dixon (1979) showed that most (nearly 75%) neurons prefer slowly moving stimuli (<20°/s). This is similar to our observations in the decorticate preparation (Pallas and Finlay 1989; Razak et al. 2003). In the mouse, superficial SC neurons respond best to stimuli moved slowly through the RF (Drager and Hubel 1975). In rats, Fukuda and Iwama (1978) showed that most superficial layer neurons responded poorly to velocities faster than 40°/s. In the cat, however, only 23% of neurons were selective for slowly moving stimuli (Dreher and Hoffman 1973). The remaining neurons responded best to stimuli moving faster than 30°/s. This is similar to what has been observed in rhesus monkeys, in which fewer than 25% of neurons preferred slowly moving stimuli (Wallace et al. 1997). It is not immediately obvious why rodents should prefer slowly moving stimuli, but given their noncarnivorous, nocturnal lifestyle it is perhaps not surprising.
Neural mechanisms of velocity tuning in the SC
The dependency of velocity tuning on the inhibitory surround can arise from spatial and/or temporal differences in activation of excitatory and inhibitory inputs with stimuli moving at different velocities. For example, a slowly moving stimulus remains in both the excitatory and the inhibitory parts of the RF for a longer time period and a rapidly moving stimulus is present for a shorter time. Although the duration of excitatory and inhibitory postsynaptic potentials cannot be ascertained from our extracellular recordings, the stimulus velocity that generates the least amount of overlap between the arrival of excitatory and inhibitory inputs on an SC neuron will obviously generate the largest response, and the stimulus velocity that generates the most overlap would produce the smallest response (Fig. 15). In LP neurons, occluding the nasal surround allowed the neuron to respond to rapidly moving stimuli, suggesting that inhibition from the nasal surround normally arrives in time to prevent or reduce the response to high-velocity stimuli (Fig. 15A). Such backward inhibition could occur if the latency of response to inhibitory input (LI) is shorter than the latency of response to the excitatory input (LE). For slowly moving stimuli, however, the stimulus stays in the eRF long enough for excitation to occur before inhibition. These properties together could give rise to a preference for slowly moving stimuli.
Fig. 15.
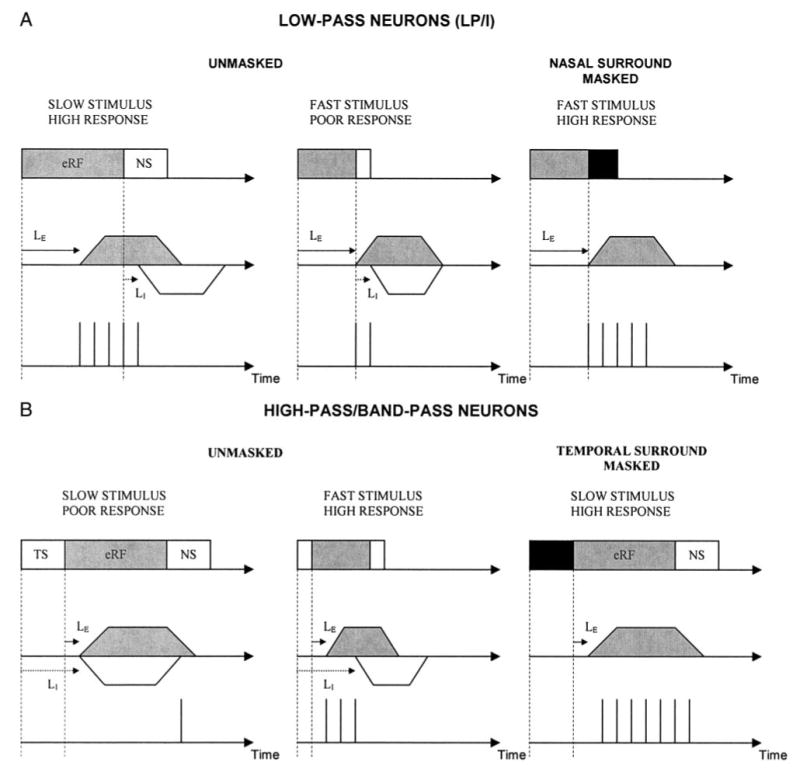
Proposed model of the spatiotemporal interactions that could underlie velocity selectivity in the SC. Differences in the latency of excitatory (LE: solid arrow) and inhibitory (LI: dotted arrow) inputs, in combination with the differences in time spent in the excitatory (gray box) and inhibitory (white box) RF by stimuli moving at different velocities predicts the observed results. Vertical lines in the 3rd row of each section of the figure each illustrate a single action potential. Trapezoids in the 2nd row of each section illustrate postsynaptic potentials (gray: excitatory; white: inhibitory). See DISCUSSION for details. Inhibition-based mechanisms of LP tuning are proposed only for the LP/I neurons because the DS neurons did not require surround inhibition for velocity tuning. Gray rectangle: excitatory RF; white rectangle: inhibitory surround; gray trapezoid: excitatory response; white trapezoid: inhibitory response.
In HP/BP neurons, occluding the temporal surround revealed a response to low-velocity stimuli not seen in the unoccluded condition (Fig. 15B). If LI is longer than LE, a temporonasally directed, slowly moving stimulus would spend sufficient time in the temporal inhibitory surround for inhibition to arrive simultaneously with or even before excitation (forward inhibition), resulting in a poor response. Occluding the TS would therefore produce an enhanced response. A high-velocity stimulus spends less time in the temporal surround before causing excitation, resulting in the late arrival of inhibition and leaving the excitatory response nearly intact.
A potential anatomical substrate for our proposed model of velocity tuning may lay in the interactions between W-type and Y-type retinal ganglion cell (RGC) inputs (Bhide and Frost 1991; Waleszczyk et al. 1999; Wang et al. 2001). The W-type RGCs described in rodents are typically tuned to slowly moving stimuli and have lower conduction velocities (King and Johnson 1985; Mooney and Rhoades 1990; Rhoades and Chalupa 1979). On the other hand, the Y-type RGCs respond well to rapidly moving stimuli, have faster conduction velocities, and typically exhibit an inhibitory surround (Mooney and Rhoades 1990). Inhibition from a neuron receiving excitatory input from a Y-type RGC onto a neuron receiving excitation from a W-type RGC may arrive earlier and result in low-pass tuning through forward masking. Inhibition from a neuron receiving excitatory input from a W-type RGC onto a neuron receiving excitation from a Y-type RGC may arrive later and suppress responses to slowly moving stimuli, creating high-pass and band-pass tuning through backward masking.
Inhibition of the responses to visual stimuli was observed when a second spot of light was swept simultaneously within the excitatory RF of some neurons (e.g., Fig. 2C). It has been suggested that the interactions between inhibition and excitation within the RF contribute to stimulus size tuning in the superior colliculus (Stein and Dixon 1979). It is also possible that these interactions play a role in velocity tuning. Our masking paradigm does not allow us to block within-eRF inhibition. Pharmacological blockade of inhibitory receptors in the SC is currently being used in our laboratory to determine whether inhibition within the eRF contributes to velocity tuning (V Khoryevin, KA Razak, and SL Pallas, unpublished observations). Additionally, blockade of inhibition within the SC will address whether the surround inhibition that contributes to velocity tuning arises within the SC or from another source, such as the retinal circuitry.
Role of inhibition in stimulus velocity tuning in the visual system
The possibility that backward masking (retroactive inhibition) contributes to velocity tuning was first proposed by Goodwin and Henry (1978) for neurons in primary visual cortex. The suggestion that inhibition plays a role in velocity tuning was supported by iontophoretic blockade of GABAA receptors (Patel and Sillito 1978). Duysens et al. (1985a,b), however, questioned the relative importance of inhibition in sculpting velocity tuning in the cat visual cortex and lateral geniculate nucleus. In that study, only 25% of visual cortical neurons were affected by masking different parts of the RF. Our study suggests that the differences observed between the previous cortical studies and our SC study could result from a difference in proportion or sampling of neurons with strong or weak inhibitory subregions in their RFs. Cortical high-pass tuning arises from two different mechanisms. First, facilitatory interactions within the excitatory RF give rise to selectivity for rapidly moving stimuli (Duysens et al. 1985). Second, temporal factors play a critical role. For example, neurons that respond well to stationary flashes of short duration also respond well to rapidly moving stimuli, whereas neurons that require a long-duration flash respond only to slowly moving stimuli (Duysens et al. 1985). Unlike cortical HP neurons, > 75% of the HP neurons in the SC depended strongly on inhibition to shape velocity tuning. These findings suggest that facilitation, duration selectivity, or inhibition can underlie stimulus velocity tuning, and the dependency on inhibition likely varies with visual processing region and with velocity preference.
Construction of complex receptive field properties in the visual system
In the visual cortex, the relative contribution of feed-forward afferent summation and intrinsic inhibitory circuits to the construction of response properties is a topic of considerable interest. Although experimental manipulation of intrinsic inhibitory circuitry has been instructive in developing models of stimulus tuning, manipulating feed-forward connections to determine the precise role of afferent convergence has not been possible. The hamster retinocollicular pathway facilitates experimental manipulation of both feed-forward convergence and inhibitory circuitry. Because hamster retinocollicular connections form after birth, and convergence of the proper number and visual field extent of afferents is dependent on NMDA receptors, chronically blocking NMDA receptors during post-natal development increases the convergence of retinal axons on SC neurons (Huang and Pallas 2001; Razak et al. 2003). Moreover, partial lesion of the caudal SC combined with NMDA receptor blockade more than doubles the visual field extent of afferent inputs from the retina onto single SC neurons (Huang and Pallas 2001). We have previously shown that this experimental increase in afferent convergence has no effect on velocity tuning (Razak et al. 2003). Taken together with the present study, these results suggest that velocity tuning in the SC is more dependent on intrinsic inhibitory circuits than on summation of a precise number of feed-forward inputs.
One striking observation from the current study is the high degree of covariance between temporal response properties and inhibitory surround properties. Most neurons with weak surround inhibition exhibited relatively tonic responses (DS neurons), whereas most neurons with strong surround inhibition exhibited phasic responses and weak stimulus duration selectivity (LP/I and HP neurons), with BP neurons representing an intermediate category. The mechanisms that generate such specificity are unclear. The fact that experimentally increasing afferent convergence fails to alter stimulus velocity tuning of SC neurons suggests that the mechanism for maintaining tuning specificity is present at a very early age and does not depend on NMDA receptor activity. This would be advantageous from a developmental perspective because during this stage in normal animals, the precise number of afferent inputs is undergoing considerable refinement. We would predict that the independence of stimulus velocity selectivity and the number of retinocollicular afferents could provide a mechanism that permits developmental or evolutionary changes in sensory circuitry, whereas other essential response properties are maintained.
In conclusion, inhibition from the surround is necessary for creating the stimulus velocity tuning seen in 65% of SC neurons. Taken together with our previous finding that increasing the convergence of retinal inputs on SC neurons has no effect on their velocity tuning (Razak et al. 2003), the results of this paper suggest a model for mechanisms of velocity tuning that depends more on inhibition than afferent convergence. There is considerable evidence that the plasticity of inhibitory inputs underlies developmental or injury-induced regulation of properties such as single-neuron response magnitude (Turrigiano 1999). It may now be possible to address the relative extent to which excitatory and inhibitory circuitry are influenced by postnatal experience in the context of circuits underlying complex response properties.
Acknowledgments
We thank Dr. Paul Katz and the members of the Pallas laboratory for critically reviewing the manuscript. We also thank Drs. Zoltan Fuzessery and Norberto Grzywacz for helpful discussions. We are grateful to K. Christian of Rockefeller University, S. Gray of Cambridge Electronic Design, and Dr. Michael Paradiso of Brown University for help with configuring stimulus generation and data acquisition software. Finally, we thank C. Paisley, C. Marshall, D. Marshall, and C. Smeeton for excellent animal care.
Present address of K. A. Razak: Dept. of Zoology and Physiology, Biological Sciences Bldg. 410, University of Wyoming, Laramie, WY 82071.
Footnotes
GRANTS
This work was supported by grants from the National Eye Institute (EY-12696), the National Science Foundation (IBN-0078110), and the Georgia State University Research Foundation to S. L. Pallas.
References
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells respond selectively to direction and speed of image motion in the rabbit. J Physiol. 1964;173:377– 407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide PG, Frost DO. Stages of growth of hamster retinofugal axons: implications for developing axonal pathways with multiple targets. J Neurosci. 1991;11:485–504. doi: 10.1523/JNEUROSCI.11-02-00485.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Different roles for GABAA and GABAB receptors in visual processing in the rat superior colliculus. J Physiol. 1997;504:629 – 639. doi: 10.1111/j.1469-7793.1997.629bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa LM, Rhoades RW. Responses of visual, somatosensory, and auditory neurons in the golden hamster’s superior colliculus. J Physiol. 1977;270:595– 626. doi: 10.1113/jphysiol.1977.sp011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Hasegawa T, Saleem KS, Tanaka K. Comparison of neuronal selectivity for stimulus speed, length, and contrast in the prestriate visual cortical areas V4 and MT of the macaque monkey. J Neurophysiol. 1994;71:2269 –2280. doi: 10.1152/jn.1994.71.6.2269. [DOI] [PubMed] [Google Scholar]
- Dawis S, Shapley R, Kaplan E, Tranchina D. The receptive field organization of X-cells in the cat: spatiotemporal coupling and asymmetry. Vision Res. 1984;24:549 –564. doi: 10.1016/0042-6989(84)90109-3. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975;38:690 –713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Dreher B, Hoffmann KP. Properties of excitatory and inhibitory regions in the receptive fields of single units in the cat’s superior colliculus. Exptl Brain Res. 1973;16:334 –353. doi: 10.1007/BF00233427. [DOI] [PubMed] [Google Scholar]
- Duysens J, Orban GA, Cremieux J. Velocity selectivity in the cat visual system. II. Independence from interactions between different loci. J Neurophysiol. 1985a;54:1050 –1067. doi: 10.1152/jn.1985.54.4.1050. [DOI] [PubMed] [Google Scholar]
- Duysens J, Orban GA, Cremieux J, Maes H. Velocity selectivity in the cat visual system. III. Contribution of temporal factors. J Neurophysiol. 1985b;54:1068 –1083. doi: 10.1152/jn.1985.54.4.1068. [DOI] [PubMed] [Google Scholar]
- Ewert JP. The neural basis of visually guided behavior. Sci Am. 1974;230:34 – 42. doi: 10.1038/scientificamerican0374-34. [DOI] [PubMed] [Google Scholar]
- Ferster D, Miller KD. Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci. 2000;23:441– 471. doi: 10.1146/annurev.neuro.23.1.441. [DOI] [PubMed] [Google Scholar]
- Fortin S, Chabli A, Dumont I, Shumikhina S, Itaya SK, Molotchnikoff Maturation of visual receptive field properties in the rat superior colliculus. Dev Brain Res. 1999;112:55– 64. doi: 10.1016/s0165-3806(98)00157-6. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Iwama K. Visual receptive-field properties of single cells in the rat superior colliculus. Jpn J Physiol. 1978;28:385– 400. doi: 10.2170/jjphysiol.28.385. [DOI] [PubMed] [Google Scholar]
- Goodwin AW, Henry GH. The influence of stimulus velocity on the responses of single neurones in the striate cortex. J Physiol. 1978;277:467– 482. doi: 10.1113/jphysiol.1978.sp012285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Berman N, Murphy EH. Effects of visual cortical lesions on receptive-field properties of single units in superior colliculus of the rabbit. J Neurophysiol. 1982;47:272–286. doi: 10.1152/jn.1982.47.2.272. [DOI] [PubMed] [Google Scholar]
- Hamasaki DI, Cohen HI. Differential response of X and Y ganglion cells to moving stimuli results from a difference in the surround inhibition. Brain Res. 1977;122:157–161. doi: 10.1016/0006-8993(77)90673-4. [DOI] [PubMed] [Google Scholar]
- Hess R, Wolters W. Responses of single cells in cat’s lateral geniculate nucleus and area 17 to the velocity of moving visual stimuli. Exp Brain Res. 1979;34:273–286. doi: 10.1007/BF00235673. [DOI] [PubMed] [Google Scholar]
- Huang L, Pallas SL. NMDA antagonists in the superior colliculus prevent developmental plasticity but not visual transmission or map compression. J Neurophysiol. 2001;86:1179 –1194. doi: 10.1152/jn.2001.86.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H, editor. Comparative Neurology of the Optic Tectum. New York: Plenum Press; 1984. [Google Scholar]
- King AJ, Johnson MS. The synaptic distribution of the retinal input in the superficial layers of the guinea-pig superior colliculus. Proc R Soc Lond B Biol Sci. 1985;225:129 –146. doi: 10.1098/rspb.1985.0055. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Functional organization of speed tuned neurons in visual area MT. J Neurophysiol. 2003;89:246 –256. doi: 10.1152/jn.00097.2002. [DOI] [PubMed] [Google Scholar]
- Lomber SG. Learning to see the trees before the forest: reversible deactivation of the superior colliculus during learning of local and global visual features. Proc Natl Acad Sci USA. 2002;99:4049 – 4054. doi: 10.1073/pnas.062551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P. Role of the superior colliculus in analyses of space: superficial and intermediate layer contributions to visual orienting, auditory orienting, and visuospatial discriminations during unilateral and bilateral deactivations. J Comp Neurol. 2001;441:44 –57. doi: 10.1002/cne.1396. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Distributed spatial coding in the superior colliculus: a review. Vis Neurosci. 1991;6:3–13. doi: 10.1017/s0952523800000857. [DOI] [PubMed] [Google Scholar]
- McIlwain JT, Buser P. Receptive fields of single cells in the cat’s superior colliculus. Exp Brain Res. 1968;5:314 –325. doi: 10.1007/BF00235906. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Ramoa AS. Intrinsic circuitry of the superior colliculus: pharmacophysiological identification of horizontally oriented inhibitory interneurons. J Neurophysiol. 1998;79:1597–1602. doi: 10.1152/jn.1998.79.3.1597. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219 –248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- Mooney RD, Rhoades RW. Relationships between physiological and morphological properties of retinocollicular axons in the hamster. J Neurosci. 1990;10:3164 –3177. doi: 10.1523/JNEUROSCI.10-09-03164.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA. The velocity tuning of single units in cat striate cortex. J Physiol. 1975;249:445– 468. doi: 10.1113/jphysiol.1975.sp011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Hoffman K-P, Duysens J. Velocity selectivity in the cat visual system. I. Responses of LGN cells to moving bar stimuli: a comparison with cortical areas 17 and 18. J Neurophysiol. 1985;54:1026 –1049. doi: 10.1152/jn.1985.54.4.1026. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kennedy H, Bullier J. Velocity sensitivity and direction selectivity of neurons in areas V1 and V2 of the monkey: influence of eccentricity. J Neurophysiol. 1986;56:462– 480. doi: 10.1152/jn.1986.56.2.462. [DOI] [PubMed] [Google Scholar]
- Orban GA, Kennedy H, Maes H. Response to movement in areas 17 and 18 of the cat: velocity sensitivity. J Neurophysiol. 1981;45:1043–1058. doi: 10.1152/jn.1981.45.6.1043. [DOI] [PubMed] [Google Scholar]
- Pallas SL, Finlay BL. Conservation of receptive field properties of superior colliculus cells after developmental rearrangements of retinal input. Vis Neurosci. 1989;2:121–135. doi: 10.1017/s0952523800011986. [DOI] [PubMed] [Google Scholar]
- Patel HH, Sillito AM. Inhibition and velocity tuning in the cat visual cortex [Proceedings] J Physiol. 1978;284:113P–114P. [PubMed] [Google Scholar]
- Razak KA, Huang L, Pallas SL. NMDA receptor blockade in the superior colliculus increases receptive field size without altering velocity and size tuning. J Neurophysiol. 2003;90:110 –119. doi: 10.1152/jn.01029.2002. [DOI] [PubMed] [Google Scholar]
- Razak KA, Pallas SL. Plasticity in inhibitory circuitry maintains velocity tuning of superior colliculus neurons under NMDA receptor blockade. Soc Neurosci Abstr. 2003;29:37–24. [Google Scholar]
- Rhoades RW, Chalupa LM. Functional properties of the corticotectal projection in the golden hamster. J Comp Neurol. 1978a;180:613– 634. doi: 10.1002/cne.901800312. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Functional and anatomical consequences of neonatal visual cortical damage in superior colliculus of the golden hamster. J Neurophysiol. 1978b;41:1466 –1494. doi: 10.1152/jn.1978.41.6.1466. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Effects of neonatal cortical lesions upon directional selectivity in the superior colliculus of the golden hamster. Brain Res. 1978c;147:188 –193. doi: 10.1016/0006-8993(78)90787-4. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Chalupa LM. Conduction velocity distribution of the retinal input to the hamster’s superior colliculus and a correlation with receptive field characteristics. J Comp Neurol. 1979;184:243–263. doi: 10.1002/cne.901840203. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Grupp LA, Pisa M. Inhibitory effect of remote visual stimuli on visual responses of cat superior colliculus: spatial and temporal factors. J Neurophysiol. 1974;37:1262–1275. doi: 10.1152/jn.1974.37.6.1262. [DOI] [PubMed] [Google Scholar]
- Shapley R, Hawken M, Ringach DL. Dynamics of orientation selectivity in the primary visual cortex and the importance of cortical inhibition. Neuron. 2003;38:689 – 699. doi: 10.1016/s0896-6273(03)00332-5. [DOI] [PubMed] [Google Scholar]
- Sillito AM. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975;250:305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9:698 –707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Stein BE, Dixon JP. Properties of superior colliculus neurons in the golden hamster. J Comp Neurol. 1979;183:269 –284. doi: 10.1002/cne.901830205. [DOI] [PubMed] [Google Scholar]
- Tiao Y-C, Blakemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol. 1976;168:483–504. doi: 10.1002/cne.901680404. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Vanegas H. The Comparative Neurology of the Optic Tectum. New York: Plenum Press; 1984. [Google Scholar]
- Waleszczyk WJ, Wang C, Burke W, Dreher B. Velocity response profiles of collicular neurons: parallel and convergent visual information channels. Neuroscience. 1999;93:1063–1076. doi: 10.1016/s0306-4522(99)00190-6. [DOI] [PubMed] [Google Scholar]
- Wallace MT, McHaffie JG, Stein BE. Visual response properties and visuotopic representation in the newborn monkey superior colliculus. J Neurophysiol. 1997;78:2732–2741. doi: 10.1152/jn.1997.78.5.2732. [DOI] [PubMed] [Google Scholar]
- Wallace MT, Meredith MA, Stein BE. Multisensory integration in the superior colliculus of the alert cat. J Neurophysiol. 1998;80:1006 –1010. doi: 10.1152/jn.1998.80.2.1006. [DOI] [PubMed] [Google Scholar]
- Wang C, Waleszczyk WJ, Benedek G, Burke W, Dreher B. Convergence of Y and non-Y channels onto single neurons in the superior colliculi of the cat. Neuroreport. 2001;2:2927–2933. doi: 10.1097/00001756-200109170-00035. [DOI] [PubMed] [Google Scholar]
- Wickelgren BG, Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969;32:16 –23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Optican LM. Superior colliculus cell types and models of saccade generation. Curr Opin Neurobiol. 1994;4:857– 861. doi: 10.1016/0959-4388(94)90134-1. [DOI] [PubMed] [Google Scholar]


