Summary
Muscle-specific kinase (MuSK) is a receptor tyrosine kinase expressed exclusively in skeletal muscle, where it is required for formation of the neuromuscular junction (NMJ). MuSK is activated by agrin, a neuron-derived heparan sulfate proteoglycan. Here, we report the crystal structure of the agrin-responsive first and second immunoglobulin-like domains (Ig1-2) of the MuSK ectodomain at 2.2 Å resolution. The structure reveals that MuSK Ig1 and Ig2 are Ig-like domains of the I-set subfamily, which are configured in a linear, semi-rigid arrangement. In addition to the canonical internal disulfide bridge, Ig1 contains a second, solvent-exposed disulfide bridge, which our biochemical data indicate is critical for proper folding of Ig1 and processing of MuSK. Two Ig1-2 molecules form a non-crystallographic dimer that is mediated by a unique hydrophobic patch on the surface of Ig1. Biochemical analyses of MuSK mutants introduced into MuSK-/- myotubes demonstrate that residues in this hydrophobic patch are critical for agrin-induced MuSK activation.
Keywords: agrin; crystal structure; immunoglobulin; MuSK; neuromuscular junction, receptor tyrosine kinase
Introduction
Formation of the vertebrate neuromuscular junction (NMJ) is guided by the exchange of signals between innervating motor neurons and muscle cells, resulting in a highly specialized postsynaptic membrane and differentiated nerve terminal, which are spatially juxtaposed.1 Neuromuscular synapse formation depends upon agrin, a large (>200 kDa), multidomain heparan sulfate proteoglycan that is secreted by motor axons and becomes stably localized in the synaptic basal lamina.2 Agrin stimulates postsynaptic differentiation by activating the muscle-specific kinase (MuSK), a receptor tyrosine kinase (RTK) expressed exclusively in skeletal muscle.3-5 Agrin and MuSK are critical for proper synaptic development, as both agrin-deficient and MuSK-deficient mice lack mature NMJs and consequently die at birth due to a failure to breathe.6,7 Downstream of agrin-induced MuSK activation, muscle-derived proteins including acetylcholine receptors (AChRs), rapsyn, ErbBs, and MuSK itself, are redistributed to the postsynaptic site and become stably localized in clusters beneath the nerve terminal.6,8 In addition, agrin-induced MuSK activation leads to selective transcriptional upregulation of synapse-specific genes by subsynaptic nuclei, and to induction of a retrograde signal leading to presynaptic differentiation.6
Other members of the RTK family include the receptors for growth factors such as the fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and nerve growth factor (NGF) (TrkA receptor). Typically, an RTK is activated through direct binding of its cognate ligand to the receptor ectodomain, which induces receptor dimerization (or higher-order oligomerization) and trans-autophosphorylation of tyrosine residues in the cytoplasmic, tyrosine kinase-containing domain.9,10
Unlike other ligand-RTK pairs, a direct interaction between agrin and the MuSK ectodomain has not been demonstrated.5 However, agrin is still regarded as the ligand for MuSK based on several observations. First, agrin stimulates the phosphorylation and kinase activity of MuSK in cultured myotubes with kinetics characteristic of ligand-receptor pairs.5 Second, cultured muscle cells lacking MuSK and cells expressing a kinase-dead/dominant-negative mutant MuSK are unable to form AChR clusters in response to agrin treatment.5,11 Lastly, agrin- and MuSK-deficient mice have very similar phenotypes.6,7 Despite such evidence supporting MuSK as the receptor for agrin, the failure to demonstrate a direct interaction between the two proteins has raised the possibility that an additional myotube-associated specificity component (MASC), such as a co-ligand, co-receptor, or myotube-specific posttranslational modification, is required for agrin to bind and activate MuSK.5 This hypothesis is supported by the observation that when MuSK is ectopically expressed in fibroblasts or myoblasts, agrin treatment fails to stimulate MuSK phosphorylation.5
The ectodomain of MuSK comprises four globular domains: three N-terminal immunoglobulin-like (Ig) domains3,4 and a C-terminal cysteine-rich region similar to the cysteine-rich domain (CRD) of Frizzled (Fz), the receptor for Wnt.12,13 Ig-like domains in other RTKs, including FGF receptors, TrkA (NGF receptor), and VEGF receptor-1, serve as the ligand binding site.14 Earlier studies aimed at identifying the domains within the MuSK ectodomain that are critical for agrin-induced AChR clustering demonstrated that the first and second Ig-like domains (Ig1-2) of MuSK are sufficient to rescue AChR clustering in MuSK-/- myotubes, suggesting that the binding site for agrin and/or the putative co-receptor resides within these two domains of MuSK.15
The lack of a direct interaction between agrin and MuSK in vitro (ref. 5 and data not shown), along with the dependence on multiple domains of agrin for MuSK activation8 and maximal AChR clustering,16-18 makes co-crystallization of agrin with the MuSK ectodomain problematic. Therefore, in an attempt to gain insights into the mechanism by which MuSK is activated by agrin, we have determined the crystal structure of Ig1-2 from the MuSK ectodomain alone. Our structural and biochemical data reveal that Ig1 possesses unique properties that are important for responsiveness to agrin and for receptor processing.
Results and Discussion
Crystal structure of MuSK Ig1-2
Ig1-2 of the rat MuSK ectodomain was expressed in a baculovirus/insect cell system. Crystals were obtained in space group P21212, with two Ig1-2 molecules in the asymmetric unit. The structure was determined by molecular replacement (see Materials and Methods) and refined at 2.2 Å resolution. Data collection and refinement statistics are given in Table 1. The crystal structure reveals that both Ig1 and Ig2 belong to the intermediate set (I-set) of the immunoglobulin superfamily (Figure 1(a)).19 In I-set Ig-like domains, two anti-parallel β sheets, one containing four β strands (ABED) and the other containing five (A‘GFCC’), are linked by an internal disulfide bridge between βB and βF, forming a β sandwich. The I-set is also characterized by a 20-residue sequence profile.19 MuSK Ig1 contains 18 of the 20 I-set profile residues (diverging at Glu-42 and Gly-113), while Ig2 contains all 20 residues.
Table 1.
X-Ray data collection and refinement statistics
| Data collection | |
|---|---|
| Resolution (Å) | 50.0-2.20 |
| Observations | 98,037 |
| Unique reflections | 27,127 |
| Completenessa (%) | 98.7 (97.5) |
| Rsyma,b (%) | 6.0 (33.1) |
| 15.9 | |
| (4.2) | |
| Refinement | |
| Number of atoms | |
| Protein | 2830 |
| Water | 178 |
| Sulfate ions | 15 (3 ions) |
| Resolution (Å) | 30.0-2.20 |
| Reflections | 24,821 |
| Rcryst/Rfreec (%) | 21.6 / 25.8 |
| Rmsd values | |
| Bond lengths (Å) | 0.010 |
| Bond angles (°) | 1.3 |
| B factorsd (Å2) (backbone / side chain) | 0.75 / 1.73 |
| Average B factors (Å2) | |
| All atoms | 31.9 |
| Protein | 31.9 |
| Water | 31.1 |
| Sulfate ions | 40.8 |
| Ramachandran plot statistics | |
| Most favored (%) | 89.5 |
| Additionally allowed (%) | 10.5 |
| Generously allowed (%) | 0 |
| Disallowed (%) | 0 |
The overall value is given first, with the value in the highest resolution shell (2.28-2.20 Å) given in parenthesis.
Rsym = 100 × Σ|I-<I>|/ΣI.
Rcryst = 100 × Σ||Fo| - |Fc||/Σ|Fo|, where Fo and Fc are the observed and calculated structure factors, respectively (Fo > 0 σ). Rfree was determined from ∼5% of the data (1309 reflections).
For bonded atoms.
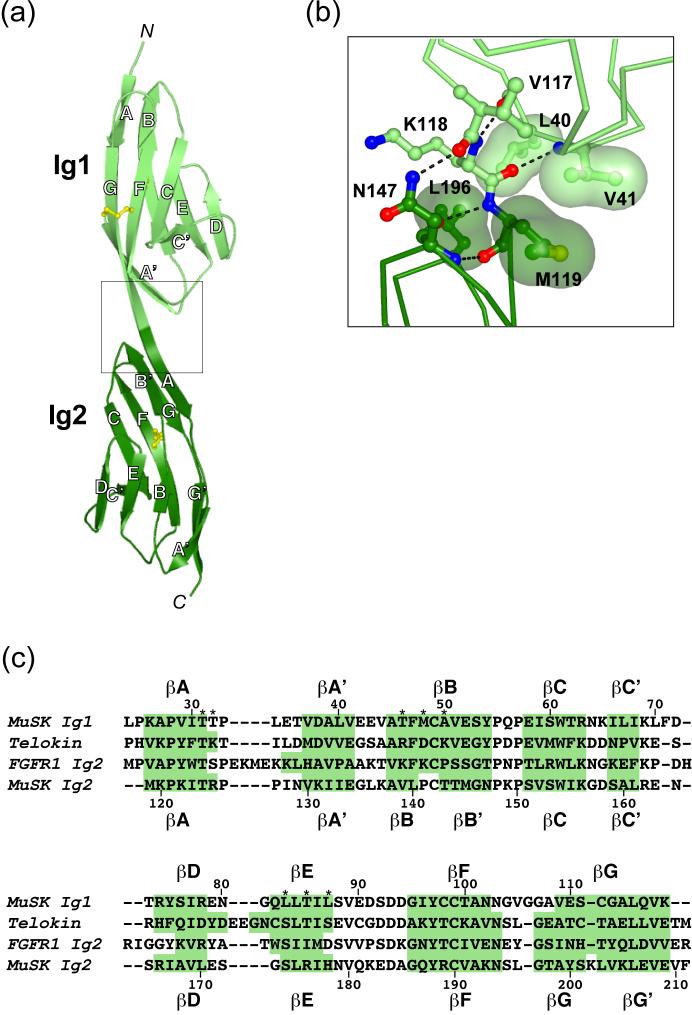
Figure 1.
Crystal structure of MuSK Ig1-2. (a) Ribbon diagram of MuSK Ig1-2. Ig1 is colored light green and Ig2 is colored dark green. The β strands are labeled, as are the N- and C-termini (N and C, respectively). Cysteine side chains are shown in ball-and-stick representation and colored yellow. The box shows the region highlighted in (b). (b) Ig1-Ig2 domain interface. Residues involved in interdomain contacts are shown in stick representation on top of a Cα trace. Oxygen atoms are colored red, nitrogen atoms blue, sulfur atoms yellow, and carbon atoms light green (Ig1) or dark green (Ig2). Hydrogen bonds are depicted as dashed black lines. Van der Waals surfaces (semi-transparent) are shown for selected side chains. (c) Structure-based sequence alignment of select I-set Ig-like domains. Shown are the sequences for MuSK Ig1 and Ig2 (rat), telokin (turkey, PDB code 1FHG20), and FGF receptor-1 (FGFR1) domain 2 (human, PDB code 1CVS42). Structural alignment was achieved using Dali.43 Residues in β strands, as determined by PROCHECK,44 are highlighted in green, and the identity of each strand is indicated above and below the sequences. Residue numbering is for MuSK Ig1 (above) and Ig2 (below), with every tenth residue numbered. Residues located in the Ig1-mediated dimer interface are marked with an asterisk (*). The V-frame nomenclature used in the text derives from Harpaz and Chothia.19 For example, residue A’B1 is the first residue in the A’B loop, and G6 is the sixth residue in βG. Figures 1(a) and 1(b) were rendered with PyMOL (http://pymol.sourceforge.net).
Ig1 superimposes onto telokin (PDB code 1FHG20), its closest structural neighbor and prototypical I-set member, with a root mean square deviation (r.m.s.d.) of 1.2 Å between equivalent Cα atoms (92 residues, 30% identity). The nearest structural neighbor to Ig2 is Ig4 of axonin-1 (PDB code 1CS621), with an r.m.s.d. of 1.3 Å for equivalent Cα atoms (89 residues, 31% identity). Also, Ig1 and Ig2 superimpose onto each other with an r.m.s.d. of 1.4 Å (90 residues, 29% identity).
An intriguing feature of MuSK Ig1 is the presence of a second disulfide bridge (in addition to the canonical internal disulfide bridge), which is on the surface of the domain and is formed by Cys-98 and Cys-112 on neighboring strands βF and βG (Figures 1(a) and 2(c), right). Cysteine residues at these positions in an Ig-like domain are unique to MuSK Ig1 (see Figure 1(c) for alignment), yet are conserved in MuSK from Torpedo californica to human, reflecting their potential functional importance. An exposed cross-strand disulfide bridge at the same position is also found in fibronectin type III domains (which are topologically similar to Ig-like domains) in class 2 cytokine receptors, including interferon receptors and tissue factor.22-24 In MuSK Ig1, the cross-strand disulfide bridge does not affect the overall fold of Ig1, nor does it alter the local structure of βF and βG.
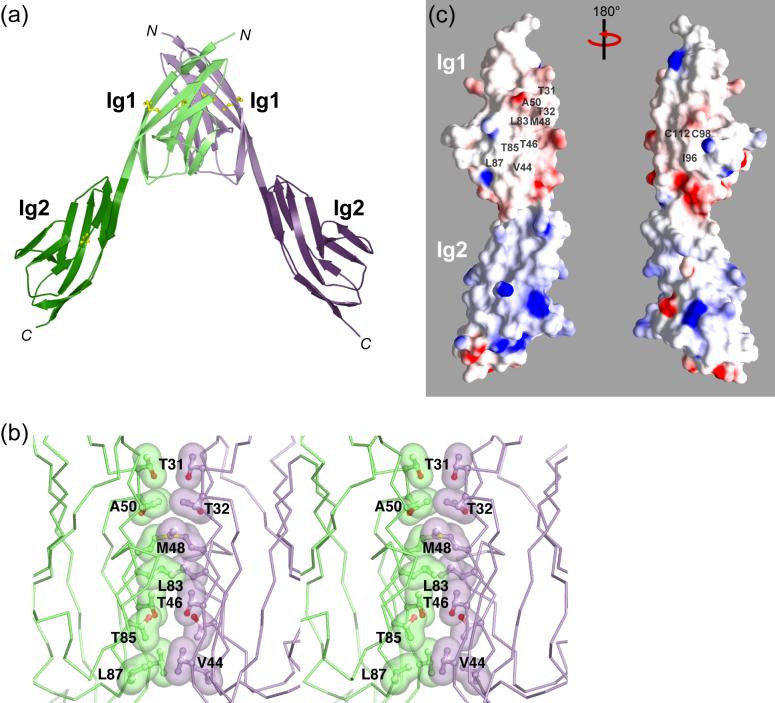
Figure 2.
MuSK Ig1-2 dimer. (a) Ribbon diagram of the MuSK Ig1-2 non-crystallographic dimer. The two protomers are colored green and purple (Ig1 in light shades and Ig2 in dark shades). The non-crystallographic two-fold axis is vertical. (b) Ig1-Ig1 dimer interface. The stereo view is approximately 50° about the vertical (two-fold) axis from that in (a). Side-chain atoms in the dimer interface are shown in ball-and-stick representation, and hydrophobic contacts are shown with semi-transparent van der Waals surfaces. Carbon atoms are colored either green or purple, oxygen atoms red, and sulfur atoms yellow. Side chains that are not labeled are related by the vertical two-fold axis to those that are labeled. (c) Molecular surface representation of Ig1-2 colored according to electrostatic potential [blue, positive (+15kT); white, neutral; red, negative (-15kT)]. Labeled are residues located in the Ig1 dimer interface (left), and in the vicinity of the surface-exposed disulfide bridge (Cys-98/Cys-112) (right), on the opposite side of Ig1. The two views are related by 180° about a vertical axis. Figures 2(a) and 2(b) were rendered with PyMOL and 2(c) with GRASP.45
Ig1 and Ig2 are disposed in a linear, head-to-tail fashion, and abut to form a rod-like molecule (Figures 1(a) and 2(c)). There is no polypeptide linker between the two domains; the final strand of Ig1 (βG) is contiguous with the first strand of Ig2 (βA) (Figure 1(b)). Interdomain contacts are present between the bottom (C-terminal) end of Ig1 and the upper (N-terminal) end of Ig2, burying 416 Å2 of surface area. The interface includes the side chains of Leu-40, Val-41 in Ig1 and Met-119, Leu-196 in Ig2, which make van der Waals contacts (Figure 1(b)). In addition, Asn-147 in Ig2 forms a hydrogen bond with the carbonyl oxygen of Val-117 in Ig1. These Ig1-Ig2 interface residues are conserved in MuSK from Torpedo to human. This interface presumably functions to preserve the linear arrangement of the two domains. Based on a sequence alignment of the MuSK Ig-like domains, we predict that Ig2 and Ig3 will also be contiguous, indicating that the first three domains of the MuSK ectodomain adopt a semi-rigid linear arrangement. Of note, a natural splice variant of MuSK contains an additional 10 residues precisely at the Ig2-Ig3 junction,4,25 which would likely introduce greater flexibility into the MuSK ectodomain. The function of this splice insert is not known at present.
In the crystal structure, a non-crystallographic dimer of Ig1-2 is present, which is mediated solely by residues in Ig1 (Figure 2(a)). The dimer interface is predominantly hydrophobic in nature, burying 1314 Å2 of total surface area with a shape complementarity (sc) value of 0.58.26 Met-48 in βB and Leu-83 in βE are at the center of the interface (Figure 2(b)). In a typical I-set Ig-like domain, the residues at these two positions are hydrophilic (e.g., Lys-177 and Ser-214, respectively, in FGF receptor-1, see Figure 1(c)), as they are solvent-exposed in a monomeric Ig-like domain (Figure 2(c), left). In MuSK, these two residues are well conserved from Torpedo to human (Met-48 is replaced by valine in chicken MuSK, and Leu-83 is replaced by isoleucine in Torpedo MuSK). Additional van der Waals contributions to the interface are made by Thr-31 and Thr-32 (AA’ loop), Val-44, Thr-46 and Ala-50 (βB), and Thr-85 and Val-87 (βE) (Figure 2(b)). Although there are no direct hydrogen bonds across the dimer interface, there are several water-mediated ones. A crystallographic dimer is present in the structure (1677 Å2 of buried surface area, sc value of 0.58), with an Ig1-Ig2 interface, but this largely hydrophilic interface is not conserved in Torpedo MuSK. Size-exclusion chromatography indicates that soluble Ig1-2 is monomeric (up to a loading concentration of 180 μM; data not shown), although this does not preclude the possibility that MuSK forms a dimer on the cell surface, where the orientation and lateral mobility of the MuSK ectodomain will be restricted due to membrane anchoring.
The two copies of Ig1 in the asymmetric unit are very similar, with an r.m.s.d. value of 0.9 Å upon superposition of all 94 Cα atoms, and of only 0.4 Å when residues of the C’D loop (69-72) are excluded. The two copies of Ig2 in the asymmetric unit are also very similar, and superimpose with an r.m.s.d. of 0.4 Å (92 Cα atoms). Comparison of the two copies of the intact Ig1-2 molecule in the asymmetric unit after superposition on Ig1 reveals that, despite the interdomain contacts described above, some flexibility is present, as the Ig2 domains in the Ig1 superposition are 22° apart, with the hinge point at Lys-118 at the end of Ig1. The C-termini of the Ig2 domains in the non-crystallographic dimer are ∼80 Å apart.
Extra disulfide in MuSK Ig1 is required for proper folding of Ig1
The structure reveals that the additional pair of cysteine residues in MuSK Ig1 (Cys-98 and Cys-112) forms a unique surface-exposed disulfide bridge. A surface-exposed disulfide bridge is also found in Ig-like domain-5 of TrkA, and is located in a hydrophobic region that provides part of the binding site for its ligand, NGF.27 To probe the function of the external disulfide bridge in MuSK Ig1, both Cys-98 and Cys-112 were mutated to serine (C98S/C112S) in a full-length MuSK-GFP fusion construct, and wild-type or mutant MuSK-GFP was stably expressed by retroviral infection in NIH-3T3 fibroblasts and MuSK-/- cultured muscle cells. We first tested whether MuSK C98S/C112S was expressed on the muscle cell surface. To this end, we labeled cell surface proteins on cultured myotubes expressing wild-type or mutant MuSK C98S/C112S with water-soluble, membrane-impermeable NHS-activated biotin, which reacts with primary amines. After biotin-labeling, the cells were lysed and biotin-conjugated proteins isolated with streptavidin-agarose beads. Biotin-labeled and total proteins were separated by SDS-PAGE and transferred to PVDF membranes. Probing Western blots with antibodies directed against cell-surface proteins such as the insulin receptor reveals that NHS-biotin effectively labels cell surface proteins, while intracellular proteins such as Shc remain unlabeled (Figure 3(a)). Unlike the wild-type receptor, MuSK C98S/C112S fails to be labeled by biotin (Figure 3(a)). We therefore conclude that the mutant receptor C98S/C112S is not expressed on the cell surface.
Figure 3.
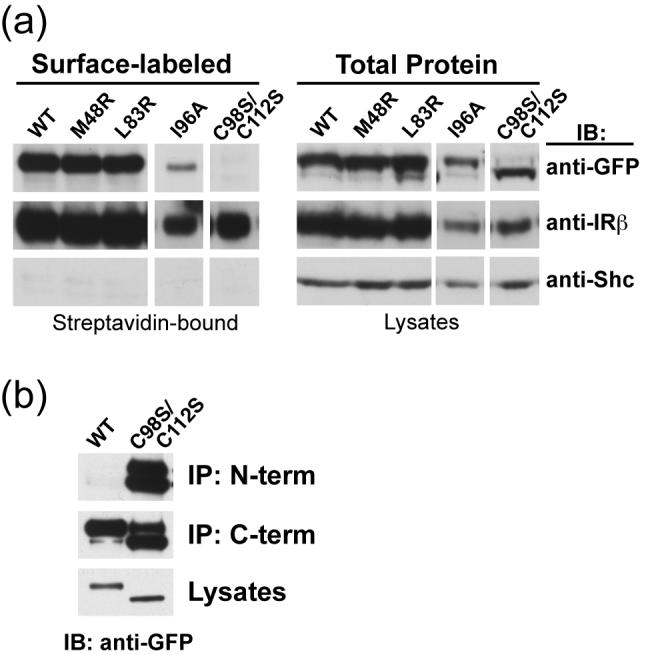
Surface expression of MuSK mutants. (a) MuSK M48R, L83R, and I96A, but not C98S/C112S, is expressed on the muscle cell surface. MuSK-GFP (wild-type or mutant) was stably expressed in cultured MuSK-/- myotubes. Cell-surface proteins were labeled with membrane-impermeable NHS-biotin and captured with streptavidin-agarose beads. Clarified lysates (2% total loaded) and surface-labeled (streptavidin-bound; 50% bound loaded) proteins were resolved by SDS-PAGE and immunoblotted (IB) with antibodies to GFP to assess the presence of MuSK in the surface fraction (anti-GFP). Immunoblotting (IB) with antibodies to the insulin receptor β subunit (IRβ) and Shc (66kDa isoform shown) confirms that cell surface proteins are effectively labeled, while cytosolic proteins remain mostly in the unlabeled fraction. The lower protein level in all I96A samples is due to fewer myotubes used as starting material. (b) Extra disulfide on MuSK Ig1 is required for proper folding and processing of MuSK. NIH-3T3 cells stably expressing wild-type or C98S/C112S mutant MuSK-GFP were lysed. MuSK immunoprecipitation (IP) was performed using polyclonal antibodies against the N-(N-term) and C-(C-term) termini. GFP-tagged MuSK was detected by immunoblotting (IB) with an anti-GFP antibody.
The failure of MuSK C98S/C112S to be expressed on the cell surface could be a consequence of improper folding and/or processing of the receptor, leading to retention of the mutant receptor in the endoplasmic reticulum or Golgi. Consistent with this hypothesis, we observe that the majority of the MuSK C98S/C112S protein migrates faster in SDS-PAGE versus wild-type MuSK, which is likely due to incomplete or no N-linked glycosylation of the mutant receptor (Figure 3(b), see anti-GFP blot of lysates). It has been shown previously that deglycosylation of MuSK by chemical treatment or asparagine mutation causes the receptor to migrate faster in SDS-PAGE.28 However, asparagine mutation of MuSK does not affect the ability of the unglycosylated receptor to be expressed on the cell surface, since the mutant receptors are activated by agrin. Therefore, it is probable that mutation of the surface-exposed disulfide bridge causes misfolding of Ig1, leading to retention of MuSK C98S/C112S inside the cell and subsequent underglycosylation of the mutant receptor. Since MuSK C98S/C112S is efficiently immunoprecipitated by an antibody against the N-terminal β strand of Ig1 (see below) and is recognized in an anti-GFP (C-terminal) immunoblot, we rule out the possibility that the change in migration of the mutant receptor on SDS-PAGE is due to proteolysis.
To assess whether removal of the additional disulfide bridge (Cys-98 and Cys-112) causes misfolding of Ig1, we tested the ability of a polyclonal antibody raised against a peptide encompassing the βA-βA’ region of Ig1 (residues 20-39) to immunoprecipitate wild-type or mutant MuSK from lysates of NIH-3T3 cells and myotubes. We find that MuSK C98S/C112S, but not wild-type, can be efficiently immunoprecipitated from lysates of both cell types by this N-terminal polyclonal antibody (Figure 3(b) and data not shown), although both receptors are detected by this antibody by immunoblot analysis in the denaturing conditions of SDS-PAGE. This result suggests that the N-terminal epitope, which is otherwise masked in the properly folded, wild-type Ig1, is exposed when the extra disulfide bridge is not present. Taken together, these data indicate that the additional disulfide bridge is required for the proper folding of MuSK Ig1. This contrasts with the surface-exposed disulfide in TrkA, which is not required for the structural integrity of Ig-like domain-5.29
Met-48, Leu-83 and Ile-96 are critical for agrin-induced MuSK activation
Proximal to the external disulfide bridge on Ig1 is Ile-96 in βF (Figure 2(c), right), which occupies a solvent-exposed position that is typically a hydrophilic residue in other Ig-like domains (e.g., Asn-227 in FGF receptor-1, Figure 1(c)). Hydrophobic residues clustered near the external disulfide bridge in TrkA contribute to ligand (NGF) binding.27 To test whether Ile-96 is involved in agrin-induced MuSK activation, we generated an alanine point mutation at Ile-96 in MuSK-GFP, and stably expressed the mutant receptor in MuSK-/- muscle cells. Figure 3(a) shows that, unlike the double cysteine mutant, the MuSK I96A mutant receptor is expressed on the surface of muscle cells. Moreover, Ig1 in the I96A mutant is evidently folded properly, since the antibody to the N-terminal β strand of Ig1 was not able to immunoprecipitate the mutant receptor from myotube lysates (data not shown).
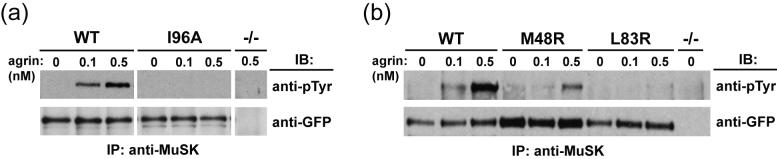
To test whether MuSK I96A is capable of being activated by agrin, we treated MuSK-/- myotubes stably expressing wild-type or mutant MuSK-GFP with recombinant neural agrin, immunoprecipitated MuSK, and probed for receptor activation by blotting with an anti-phosphotyrosine antibody. Figure 4(a) shows that, while agrin induces activation of wild-type MuSK in a dose-dependent manner, activation of MuSK I96A is completely abolished.
Figure 4.
Mutations in MuSK Ig1 affect agrin-induced receptor activation. (a) Ile-96 and (b) Met-48 and Leu-83 are required for agrin-induced activation. MuSK-/- myotubes stably expressing MuSK-GFP (wild-type or mutant) were stimulated with 0.1 or 0.5 nM neural agrin for 30 min followed by MuSK immunoprecipitation (IP) with the C-terminal anti-MuSK antibody. IP samples were resolved by SDS-PAGE and immunoblotted (IB) with an anti-phosphotyrosine antibody (anti-pTyr) to assess MuSK activation. Blotting a duplicate membrane with anti-GFP antibody shows the relative level of MuSK-GFP in each sample.
Residues Met-48 and Leu-83 in MuSK Ig1 are at the center of the non-crystallographic dimer interface (Figure 2(b)). To begin to elucidate the function of this hydrophobic patch, we generated arginine point mutations at Met-48 and Leu-83 in MuSK-GFP, and stably expressed the mutants in MuSK-/- muscle cells. As shown in Figure 3(a), the mutants are expressed on the surface of muscle cells at levels equal to wild type. Furthermore, mutation of Met-48 or Leu-83 to arginine does not cause misfolding of Ig1, as neither mutant receptor is immunoprecipitated from myotube lysates by the N-terminal β strand antibody (data not shown).
To test the ability of MuSK M48R and L83R to be activated by agrin, we treated MuSK-/- myotubes expressing wild-type or mutant MuSK-GFP with recombinant neural agrin, immunoprecipitated MuSK, and probed Western blots with anti-phosphotyrosine antibody. Figure 4(b) (top blot) shows that the level of activation of the mutant receptors M48R and L83R is severely reduced compared to the robust activation of wild-type MuSK. The decrease in agrin-induced activation of the mutant receptors occurs despite the elevated expression levels of the mutants versus wild-type MuSK, as assessed by probing with antibodies against GFP (Figure 4(b), bottom blot). The stronger phosphotyrosine signal of MuSK M48R versus L83R with 0.5 nM agrin treatment is due to a greater amount of MuSK-GFP M48R in the myotubes. Quantitative densitometry analysis of the anti-phosphotyrosine and anti-GFP immunoblots indicates that the two mutants are equally unresponsive to agrin.
On the cell surface, we anticipate that the hydrophobic patch on Ig1 will mediate a protein-protein interaction, either MuSK homodimerization (as observed in the crystal structure) or a heterotypic interaction with another protein such as agrin or a putative agrin co-receptor.5 One possible scenario is that the MuSK Ig1-2 dimer in the crystal structure represents a preformed, autoinhibited conformation of the receptor. There is evidence that some cytokine receptors (e.g., erythropoietin receptor) and RTKs (e.g, EGF receptor) exist on the cell surface as inactive dimers and undergo ligand-induced rearrangements to form an active signaling complex.30,31 It is unlikely that the Ig1-mediated MuSK dimer observed in the crystal structure is autoinhibitory, since we did not observe an increase in the basal level of tyrosine phosphorylation in the dimer-disrupting M48R and L83R mutants (Figure 4(b)).
Another possibility is that the Ig1-mediated MuSK homodimer in the crystal structure reflects an active dimeric state whose formation is induced by agrin binding. We attempted cross-linking experiments in myotubes to assay agrin binding to wild-type MuSK and to MuSK M48R and L83R. However, even with wild-type MuSK, we were unable to detect a cross-linked agrin-MuSK complex (data not shown). Therefore, we are unable to distinguish whether Met-48 and Leu-83 in the Ig1 hydrophobic patch are critical for MuSK dimerization or for binding of agrin or a putative co-receptor. Nevertheless, to our knowledge, this is the first time that individual residues in the MuSK ectodomain have been identified as critical for agrin-stimulated MuSK activation. A greater understanding of the full componentry of the agrin-MuSK signaling complex will be required to fully elucidate the mechanisms of activation for this ligand-receptor pair.
Materials and Methods
Cell culture
Sf9 cells were maintained in EX-CELL 401 medium with L-glutamine (SAFC Biosciences) supplemented with 5% fetal bovine serum (FBS), 0.1% Pluronic F-68 (Cellgro Mediatech), and antibiotic-antimycotic (10 U/ml penicillin, 10 μg/ml streptomycin (pen-strep), 25 ng/ml amphotericin B; Invitrogen) at 27°C. BOSC23 packaging cells32 were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 1 mM glutamine, 10% FBS and 50 μg/ml gentamycin. NIH-3T3 cells were grown in DMEM with 10% FBS, 1mM glutamine, 100 U/ml pen-strep and 50 μg/ml gentamycin. MuSK-/- myoblasts33 were grown at 33°C in growth medium containing DMEM plus 1 mM glutamine, 10% FBS, 10% horse serum, 50 μg/ml gentamycin, 0.5% chick embryo extract (CEE; Accurate Chemical), and 20 U/ml recombinant mouse interferon-γ (IFN-γ; Sigma) on dishes coated with Matrigel (BD Biosciences). Myoblasts at 90% confluence were induced to differentiate into myotubes by switching to 39°C in medium lacking INF-γ and CEE.
Antibodies
The polyclonal antibody to the C-terminal sequence of rat MuSK (residues 849-868) was described previously.34 For production of the polyclonal antibody to the N-terminal sequence of rat MuSK, a peptide corresponding to residues 20-39 (GTEKLPKAPVITTPLETVDA) was coupled to keyhole limpet hemocyanin and injected into rabbits (Research Genetics). The phosphotyrosine antibody mAb 4G10 and polyclonal anti-Shc antibody were purchased from Upstate. The monoclonal GFP antibody B-2 was purchased from Santa Cruz Biotechnology. The polyclonal antibody against the insulin receptor β subunit was purchased from Biosource International. Horseradish-peroxidase (HRP)-conjugated secondary antibodies were purchased from Jackson ImmunoResearch Laboratories and Biosource International.
Protein expression and purification
A PCR-generated cDNA fragment encoding MuSK Ig1-2 (residues 22-212 of rat MuSK) was ligated into the baculovirus transfer vector pAcGP67-B (BD Biosciences Pharmingen) that was modified to contain an N-terminal six-histidine tag (gift of Dr. K. Carraway, UC Davis). Recombinant baculovirus was generated by co-transfecting Sf9 cells with transfer vector and linearized BaculoGold DNA according to the manufacturer’s protocol (BD Biosciences Pharmingen). Adherent Sf9 cells were infected during log phase growth with high titer baculovirus, and media containing the secreted protein was harvested by centrifugation 72 h later. Secreted MuSK Ig1-2 protein, which is not glycosylated, was purified by nickel-affinity chromatography (HIS-Select HC; Sigma), gel-filtration and cation-exchange chromatography (Superdex 75 and Source S, respectively; GE Healthcare). MALDI-TOF analysis of purified MuSK Ig1-2 revealed a heterogeneous protein sample, due to in vitro proteolytic trimming. To obtain a homogenous minimal protein fragment, MuSK Ig1-2 was subjected to limited proteolysis by trypsin (sequencing grade; Roche), which resulted in removal of 14 residues from the N-terminus including the His6-tag, leaving the full Ig1-2 protein core intact. The sample was further purified over a Benzamidine HiTrap column (GE Healthcare) to remove trypsin, and cation exchange (Source S) to obtain a homogenous sample, as monitored by MALDI-TOF, suitable for crystallization. Purified protein was concentrated in a spin concentrator (VivaScience).
Crystallization and data collection
Crystals of MuSK Ig1-2 were grown at 4°C in hanging drops containing 1:1 ratio by volume of protein solution at 9 mg/ml and reservoir buffer containing 8% polyethylene glycol (PEG) 4000, 100 mM sodium acetate, pH 4.6, and 200 mM ammonium sulfate. (The same crystal form could be obtained at neutral pH, but the crystals were of poorer quality.) Crystals belong to orthorhombic space group P21212 with unit cell dimensions a=77.6 Å, b=118.0 Å, and c=57.8 Å. There are two Ig1-2 molecules in the asymmetric unit with a solvent content of 61%. Prior to stream freezing in liquid nitrogen, crystals were equilibrated in a series of cryosolvents containing 5%, 10%, 15%, then 20% ethylene glycol. A 2.2 Å dataset was collected on beamline X4A at the National Synchrotron Light Source, Brookhaven National Laboratory. Data were processed using DENZO/Scalepack.35 A molecular replacement solution was found with AMoRE36 with SWISS-MODEL homology models37 of MuSK Ig1 and Ig2 based on the structure of telokin (PDB code 1FHG20). Rigid-body refinement, simulated annealing, and positional and B-factor refinement were performed with CNS38 and Refmac,39 and O40 was used for model building.
Retroviral expression constructs and infection of cells
The SmaI/NotI fragment of pEGFP-N1 (Clontech) containing GFP cDNA was fused in-frame to the 3’ end of full-length wild-type rat MuSK cDNA. MuSK-GFP cDNA was ligated into the pUC18 vector (Stratagene), where amino acid substitutions in the MuSK extracellular domain were generated by site-directed mutagenesis (QuikChange; Stratagene). All mutations were verified by automated DNA sequencing. The MuSK-GFP wild-type or mutant fragments were then ligated into the EcoRI site of the retroviral vector pBabe/puro.41 Full-length wild-type or mutant MuSK-GFP was stably expressed in NIH-3T3 and MuSK-/- cells by retroviral infection. BOSC23 packaging cells were transfected with MuSK/GFP-pBabe/puro plasmids by the calcium phosphate precipitation method. Retrovirus-containing medium was collected 2 days post-transfection and used immediately for infection of NIH-3T3 and MuSK-/- cells, or flash frozen and stored at -80°C. For infection, NIH-3T3 cells and MuSK-/- myoblasts at 30-50% confluence were incubated with growth medium containing virus and 8 μg/ml polybrene for 4 h. The polybrene concentration was then diluted to 2 μg/ml by addition of growth medium, and cells were incubated overnight. 24-48 h post-infection, cells were placed under selection with medium containing 4 μg/ml puromycin and maintained under selective conditions until stable pools were formed.
MuSK activation by agrin and MuSK immunoprecipitation
For agrin-induced MuSK stimulation, fully differentiated myotubes were treated with 0.1-0.5 nM neural agrin (R&D Systems) in DMEM for 30-50 min at 37°C. For all MuSK immunoprecipitation experiments, myotubes or NIH-3T3 cells were rinsed twice with ice-cold phosphate-buffered saline (PBS) and lysed in lysis buffer containing 1% NP-40, 30 mM triethanolamine, pH 7.5, 50 mM NaCl, 5 mM EDTA, 5 mM EGTA, 50 mM NaF, 2 mM sodium orthovanadate, 1 mM sodium tetrathionate, 1 mM N-ethylmaleimide, 10 μM Pepstatin A, 0.5 mg/ml Pefabloc (Roche), and Complete protease inhibitor tablet (Roche). Whole-cell lysates were cleared by centrifugation, and MuSK protein was immunoprecipitated at 4°C overnight with the N-terminal (Figure 4(b)) or the C-terminal (Figures 4(b) and 4(c)) anti-MuSK antibodies. The antibodies and bound proteins were captured by incubating with Protein-A agarose beads (Roche) for an additional hour at 4°C. The beads were then washed four times in freshly made lysis buffer. Bound proteins were eluted with Laemmli sample buffer, resolved by SDS-PAGE, and transferred to PVDF membrane (Millipore). Membranes were blocked in TBST (Tris-buffered saline, 0.05% Tween-20) containing 5% bovine serum albumin (BSA; for anti-phosphotyrosine blots) or 5% instant skimmed milk, and probed with primary antibodies and HRP-conjugated secondary antibodies, which were visualized with SuperSignal West Pico and West Femto Substrates (Pierce).
Biotinylation of cell surface proteins
Myotubes were washed extensively with PBS containing 0.1 mM CaCl2 and 1 mM MgCl2(PBS/Ca/Mg) and incubated with 1 mM EZ-link sulfo-NHS-LC-biotin (Pierce) in PBS/Ca/Mg at room temperature for 30 min. The reaction was quenched by rinsing the cells twice with PBS/Ca/Mg containing 100 mM glycine, and incubated in DMEM at 37°C for 30 min. The cells were then placed on ice, washed with ice-cold PBS, and lysed in RIPA buffer (1% NP-40, 1% sodium deoxycholate, 150 mM NaCl, 10 mM sodium phosphate buffer, pH 7.2, 2 mM EDTA, 50 mM NaF, 2 mM sodium orthovanadate, 10 μM Pepstatin A, 0.5 mg/ml Pefabloc, and Complete protease inhibitor tablet (Roche)) containing 0.5% SDS. Lysates were cleared by centrifugation, and biotin-labeled proteins were recovered by incubating with streptavidin-agarose (Sigma) for 4 h at 4°C, followed by four washes with RIPA buffer containing 0.1% SDS. Proteins bound to streptavidin-agarose were eluted with Laemmli sample buffer, and bound and total proteins were resolved by SDS-PAGE and detected by Western blotting.
Acknowledgments
Support is acknowledged from the National Institutes of Health (NS053414 to S.R.H., NS036193 to S.J.B.). We thank M Mohammadi for helpful comments on the manuscript, G. Dorsainville and M. Friese for experimental support and discussions, R. Abramowitz for synchrotron data collection assistance, and Dr. K. Carraway for the baculovirus transfer vector.
References
- 1.Burden SJ. Building the vertebrate neuromuscular synapse. J. Neurobiol. 2002;53:501–11. doi: 10.1002/neu.10137. [DOI] [PubMed] [Google Scholar]
- 2.McMahan UJ. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990;55:407–18. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Jennings CG, Dyer SM, Burden SJ. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc. Natl. Acad. Sci. U.S.A. 1993;90:2895–9. doi: 10.1073/pnas.90.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valenzuela DM, Stitt TN, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park JS, Stark JL, Gies DR, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–84. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 5.Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, DiStefano PS, Valenzuela DM, DeChiara TM, Yancopoulos GD. Agrin acts via a MuSK receptor complex. Cell. 1996;85:513–23. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- 6.Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–35. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- 7.DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–12. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- 8.Hopf C, Hoch W. Tyrosine phosphorylation of the muscle-specific kinase is exclusively induced by acetylcholine receptor-aggregating agrin fragments. Eur. J. Biochem. 1998;253:382–9. doi: 10.1046/j.1432-1327.1998.2530382.x. [DOI] [PubMed] [Google Scholar]
- 9.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–12. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 10.Hubbard SR, Till JH. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000;69:373–98. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 11.Glass DJ, Apel ED, Shah S, Bowen DC, DeChiara TM, Stitt TN, Sanes JR, Yancopoulos GD. Kinase domain of the muscle-specific receptor tyrosine kinase (MuSK) is sufficient for phosphorylation but not clustering of acetylcholine receptors: required role for the MuSK ectodomain? Proc. Natl. Acad. Sci. U.S.A. 1997;94:8848–53. doi: 10.1073/pnas.94.16.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu YK, Nusse R. The Frizzled CRD domain is conserved in diverse proteins including several receptor tyrosine kinases. Curr. Biol. 1998;8:R405–6. doi: 10.1016/s0960-9822(98)70262-3. [DOI] [PubMed] [Google Scholar]
- 13.Rehn M, Pihlajaniemi T, Hofmann K, Bucher P. The frizzled motif: in how many different protein families does it occur? Trends Biochem. Sci. 1998;23:415–7. doi: 10.1016/s0968-0004(98)01290-0. [DOI] [PubMed] [Google Scholar]
- 14.Wiesmann C, Muller YA, de Vos AM. Ligand-binding sites in Ig-like domains of receptor tyrosine kinases. J. Mol. Med. 2000;78:247–60. doi: 10.1007/s001090000082. [DOI] [PubMed] [Google Scholar]
- 15.Zhou H, Glass DJ, Yancopoulos GD, Sanes JR. Distinct domains of MuSK mediate its abilities to induce and to associate with postsynaptic specializations. J. Cell Biol. 1999;146:1133–46. doi: 10.1083/jcb.146.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornish T, Chi J, Johnson S, Lu Y, Campanelli JT. Globular domains of agrin are functional units that collaborate to induce acetylcholine receptor clustering. J. Cell. Sci. 1999;112:1213–23. doi: 10.1242/jcs.112.8.1213. [DOI] [PubMed] [Google Scholar]
- 17.Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor-aggregating activity of agrin isoforms and mapping of the active site. J. Cell Biol. 1995;128:625–36. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoch W, Campanelli JT, Harrison S, Scheller RH. Structural domains of agrin required for clustering of nicotinic acetylcholine receptors. EMBO J. 1994;13:2814–21. doi: 10.1002/j.1460-2075.1994.tb06575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harpaz Y, Chothia C. Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains. J. Mol. Biol. 1994;238:528–39. doi: 10.1006/jmbi.1994.1312. [DOI] [PubMed] [Google Scholar]
- 20.Holden HM, Ito M, Hartshorne DJ, Rayment I. X-ray structure determination of telokin, the C-terminal domain of myosin light chain kinase, at 2.8 A resolution. J. Mol. Biol. 1992;227:840–51. doi: 10.1016/0022-2836(92)90226-a. [DOI] [PubMed] [Google Scholar]
- 21.Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P, Welte W. The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell. 2000;101:425–33. doi: 10.1016/s0092-8674(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 22.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc. Natl. Acad. Sci. U.S.A. 1990;87:6934–8. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller YA, Ultsch MH, de Vos AM. The crystal structure of the extracellular domain of human tissue factor refined to 1.7 A resolution. J. Mol. Biol. 1996;256:144–59. doi: 10.1006/jmbi.1996.0073. [DOI] [PubMed] [Google Scholar]
- 24.Walter MR, Windsor WT, Nagabhushan TL, Lundell DJ, Lunn CA, Zauodny PJ, Narula SK. Crystal structure of a complex between interferon-gamma and its soluble high-affinity receptor. Nature. 1995;376:230–5. doi: 10.1038/376230a0. [DOI] [PubMed] [Google Scholar]
- 25.Kuehn R, Eckler SA, Gautam M. Multiple alternatively spliced transcripts of the receptor tyrosine kinase MuSK are expressed in muscle. Gene. 2005;360:83–91. doi: 10.1016/j.gene.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence MC, Colman PM. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 1993;234:946–50. doi: 10.1006/jmbi.1993.1648. [DOI] [PubMed] [Google Scholar]
- 27.Wiesmann C, Ultsch MH, Bass SH, de Vos AM. Crystal structure of nerve growth factor in complex with the ligand-binding domain of the TrkA receptor. Nature. 1999;401:184–8. doi: 10.1038/43705. [DOI] [PubMed] [Google Scholar]
- 28.Watty A, Burden SJ. MuSK glycosylation restrains MuSK activation and acetylcholine receptor clustering. J. Biol. Chem. 2002;277:50457–62. doi: 10.1074/jbc.M208664200. [DOI] [PubMed] [Google Scholar]
- 29.Urfer R, Tsoulfas P, O’Connell L, Hongo JA, Zhao W, Presta LG. High resolution mapping of the binding site of TrkA for nerve growth factor and TrkC for neurotrophin-3 on the second immunoglobulin-like domain of the Trk receptors. J. Biol. Chem. 1998;273:5829–40. doi: 10.1074/jbc.273.10.5829. [DOI] [PubMed] [Google Scholar]
- 30.Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–90. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- 31.Moriki T, Maruyama H, Maruyama IN. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J. Mol. Biol. 2001;311:1011–26. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- 32.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8392–6. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst R, Burden SJ. The juxtamembrane region of MuSK has a critical role in agrin-mediated signaling. EMBO J. 2000;19:67–77. doi: 10.1093/emboj/19.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watty A, Neubauer G, Dreger M, Zimmer M, Wilm M, Burden SJ. The in vitro and in vivo phosphotyrosine map of activated MuSK. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4585–90. doi: 10.1073/pnas.080061997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 36.Navaza J. AMoRe: an automated package for molecular replacement. Acta Crystallogr. A. 1994;50:157–163. [Google Scholar]
- 37.Peitsch MC. ProMod and Swiss-Model: Internet-based tools for automated comparative protein modelling. Biochem. Soc. Trans. 1996;24:274–9. doi: 10.1042/bst0240274. [DOI] [PubMed] [Google Scholar]
- 38.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D. 1998;54:905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 39.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. 1997;53:240–55. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 40.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A. 1991;47:110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 41.Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–96. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plotnikov AN, Schlessinger J, Hubbard SR, Mohammadi M. Structural basis for FGF receptor dimerization and activation. Cell. 1999;98:641–50. doi: 10.1016/s0092-8674(00)80051-3. [DOI] [PubMed] [Google Scholar]
- 43.Holm L, Sander C. Alignment of three-dimensional protein structures: network server for database searching. Methods Enzymol. 1996;266:653–62. doi: 10.1016/s0076-6879(96)66041-8. [DOI] [PubMed] [Google Scholar]
- 44.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993;26:283–91. [Google Scholar]
- 45.Nicholls A, Sharp KA, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–96. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]