Abstract
G protein-coupled receptor (GPCR)-mediated signal transduction has been studied for more than a century. Despite the intense focus on this class of proteins, a molecular understanding of what constitutes the functional form of the receptor is still uncertain. GPCRs have traditionally been conceptualized as monomeric proteins, and this view has changed little over the years until relatively recently. Recent biochemical and biophysical studies have challenged this traditional concept, and point instead to a mechanistic view of signal transduction wherein the receptor functions as an oligomer. Cooperative interactions within such an oligomeric array may be critical for the propagation of an external signal across the cell membrane and to the G protein, and may therefore underlie the mechanistic basis of signaling.
G protein-coupled receptors (GPCRs)1 constitute by far the largest family of cell surface proteins involved in signaling across biological membranes. There are approximately 950 genes in the human genome encoding proteins in this superfamily (1). GPCRs modulate a wide range of physiological processes and are implicated in numerous diseases. They form the largest class of therapeutic targets (2, 3).
Stimuli for GPCRs are as diverse as light, odorants, neurotransmitters, and peptides; the physiological processes regulated by these proteins are likewise varied (4). Despite this variation, all GPCRs share a common seven-α-helix transmembrane architecture and presumably signal by a common mechanism via a heterotrimeric guanyl nucleotide-binding protein (G protein) (5). Nonclassical roles for GPCRs have also emerged that are independent of the G protein (6). Mammalian GPCRs are commonly divided into three distinct subfamilies based on sequence: families A–C (4, 6). More recently, GPCRs have been classified into five distinct groups based on phylogenetic analyses of sequences from the human genome (7). This classification system has been named GRAFS, which is an acronym for the five different groups: glutamate, rhodopsin, adhesion, frizzled/taste2, and secretin.
The current state of GPCR research has evolved in large measure from observations made in two parallel systems: hormone-activated receptors that regulate adenylate cyclase (AC) and light-activated rhodopsin, which regulates phosphodiesterase (Figure 1). The focus of this work is to provide an overview of the field from the early days to the present to show how the perception of GPCR oligomerization has progressed and changed over the years, and to show the potential significance of oligomerization to the mechanism of G protein-mediated signaling.
Figure 1.

Evolving molecular concept of a G protein-coupled receptor. The molecular understanding of GPCRs during different periods in the history of the field is represented for GPCRs acting via AC and for the visual pigment rhodopsin. In the AC branch, agonists (yellow) were initially thought to bind to some part of the cell or tissue (A). The receptor was next thought to be a part of AC (violet) itself (B). It was then discovered that the receptor (blue) was a distinct molecular entity (C). The receptor has been conceptualized as a monomeric protein until relatively recently. Our current understanding is that the receptor exists as an oligomer (D). In the rhodopsin branch, the initial detection of rhodopsin (red) came from its characteristic reddish-purple color in the rod outer segments (E). The color was then determined to derive from a chromophore conjugated to a protein. Early biophysical methods suggested that rhodopsin was monomeric (F). More recent studies using atomic force microscopy point to an oligomeric arrangement of rhodopsin (G).
Adenylate Cyclase Signal Transduction Pathway
Many of the classical pharmacological theories have emerged from observations made in GPCR-mediated systems (8-10). Although the concept of a receptor has been around for more than a century (11-13), the receptor, or the “receptive substance” as termed by Langley (13), was an abstract notion at best in early years. Drugs such as acetylcholine and epinephrine were known to elicit physiological responses when applied to tissue (14); however, the site of action of those drugs was unknown, and it was assumed that they bound to some part of the tissue or cell (Figure 1).
Clark was the first to attempt to quantify the action of drugs by looking at their dose–response relationships (Figure 2, Scheme 1) (8). The drugs appeared to act in accord with the Langmuir isotherm, which originally was derived from the law of mass action to describe the adsorption of gases on metal surfaces (15). Clark's theory was later modified to include properties of the receptor such as intrinsic activity and efficacy (Figure 2, Scheme 2) (9, 10, 16-18). Common to all these theories was the notion of a single site or receptor. These ideas formed the concept of a receptor long before the discovery of the actual molecular identity of the proteins responsible for producing a physiological response. And it is within this framework that we continue to view the system even as we learn more about the proteins responsible for signaling.
Figure 2.
Schemes used to describe the interaction between an agonist and its receptor. Scheme 1 describes Clark's occupancy theory (8). In Clark's theory, an agonist (A) will combine with the receptor (R) to form an agonist–receptor complex (AR). The level of the AR complex present at thermodynamic equilibrium can be defined by the equilibrium dissociation constant (KA). A physiological response (Q) will result only when the AR complex is formed, and a maximal response (Qmax) will occur when the agonist occupies all receptor sites. Scheme 2 describes the Stephenson–Furchgott model of efficacy (10). Stephenson postulated that the activity of the agonist is determined by the affinity of the drug for the receptor (KA) and efficacy (e). Since the occupancy of receptors by the agonist did not appear to be linearly proportional to the response (Q), a dimensionless quantity called the stimulus (S) was introduced. The stimulus would lead to a response in a relationship that was not apparent at the time and was therefore left undefined [i.e., Q = f(S)]. The stimulus is defined explicitly as the product of efficacy and the fraction of receptors occupied by the agonist (i.e., S = e[AR]/[R]t). This model was refined later by Furchgott, who defined efficacy as the product of the concentration of the total active receptor ([R]t) and a quantity specific to the agonist–receptor complex designated as the intrinsic efficacy (ε) [i.e., e = ε[R]t, S = ε[AR] = e[A]/(KA + [A])] (9). An explicit expression for the response is given for the simple case in which the dose–response relationship is in the form of a rectangular hyperbola. Scheme 3 describes the multisite model (154), where an agonist (A) can bind to multiple classes of sites (Rj, j = 1, 2, 3, …, n) that are mutually independent and non-interconverting. The agonist can bind to each of the sites with distinct affinities (KAj). An equation for the binding isotherm is provided where Y is the fractional occupancy of the receptor by the agonist (i.e., ) and fj is the fraction of total receptor that is of type j (i.e., fj = [Rj]t/[R]t, where . Scheme 4 describes the ternary complex model (138, 151), where the receptor (R) and G protein (G) are freely mobile within the plane of the membrane and interact through random collisions. Agonists bind to the free receptor (R) or receptor coupled to the G protein (RG) with dissociation constants KA and KAG, respectively. The G protein binds to the free receptor or receptor occupied by agonist (AR) with dissocation constants KG and KGA, respectively. Agonists promote coupling (KAG < KA) and therefore favor a ternary complex (ARG) (KA/KAG = KG/KGA). There are three conditions that need to be met for the ternary complex model to account for the dispersion of affinities observed in the binding of agonists. First, the agonist must differ in its affinity for the coupled and uncoupled states of the receptor (i.e., KA ≠ KAG). Second, the total concentration of the receptor must be bracketed by the affinity of the receptor for the G protein in the absence and presence of the agonist (i.e., KGA < [R]t < KG). Third, the total number of G proteins must be equal to or less than the total number of receptors (i.e., [G]t ≤ [R]t). If these conditions are not met, then the ternary complex model predicts that agonist binding curves will have the form of a rectangular hyperbola (i.e., nH = 1) (139, 142, 151, 153, 210). Scheme 5 describes cooperativity within a tetravalent receptor (n = 4) that interconverts spontaneously between two states (R and T) (101). The distribution of sites in the absence of agonist is defined by the constant KRT. Binding occurs in a sequential manner where the first equivalent of agonist binds the vacant oligomer with the microscopic dissociation constant KAR or KAT (aR1 = 1 and aT1 = 1; A0R ≡ R and A0T ≡ T). The degree of cooperativity exerted on the binding of subsequent equivalents of the agonist will be reflected in cooperativity factors (aRj and aTj, j = 2, 3, or 4). Positive cooperativity occurs when aRj or aTj is less than 1; negative cooperativity occurs when aRj or aTj is greater than 1, and no cooperativity occurs when aRj or aTj is equal to 1. The oligomer is assumed to be symmetric, in that two or more vacant sites within either R or T are indistinguishable (1 ≤ j ≤ 3).
The molecular identity of hormone-binding GPCRs was only revealed after the isolation and characterization of AC (19), an enzyme now known to serve as an effector for many GPCRs. The importance of AC and the discovery of its product, cAMP, came initially from studies by Sutherland and co-workers on liver phosphorylase (20-22). AC was shown to be modulated by various structurally dissimilar hormones, such as adrenocorticotropic hormone (ACTH), serotonin, thyroid-stimulating hormone (TSH), and acetylcholine (23-26). At that time, it was proposed by Sutherland that the hormone-binding site (i.e., the receptor) was actually a part of AC itself in a manner analogous to that of multisubunit enzymes, which are composed of a regulatory unit and a catalytic unit (27).
The nonadditivity in the response and the dissimilarity in the structures of the various hormones affecting AC suggested that those compounds acted at functionally distinct sites (28, 29). The ability to characterize the nature of the hormone-binding site was improved greatly when radiolabeled analogues of the hormones were introduced. One of the earliest measurements using radiolabeled probes was made in intestinal smooth muscle, in studies on the binding properties of the muscarinic agonist [14C]methylfurmethide and the antagonists [3H]atropine and [3H]methylatropine (30). It later was shown, using [125I]ACTH and [125I]glucagon, that those hormones were able to bind their respective sites in a saturable and reversible manner (31, 32). The radioligand binding site for both the glucagon receptor and the adrenergic receptor was found to elute in different fractions from AC activity in gel filtration studies (33-35). Moreover, hormone-sensitive AC activity was recovered after the fusion of a cell containing an inactive AC with another that lacked adrenergic receptors (36). These studies demonstrated the structural distinction between the receptor and the enzyme.
At about the same time, it was also becoming apparent that an additional protein was involved in propagating external stimuli (reviewed in ref 37). Largely from work in the laboratories of Rodbell and Gilman came the realization that there was a third component of the signal transduction machinery (38-40). This protein, now known as the G protein, bound GTP and had intrinsic GTPase activity. It was also becoming apparent at about this time that cGMP phosphodiesterase activity in rod outer segments of the retina was dependent on both light and guanyl nucleotides in a manner that mirrored the activation of the AC system (41). In addition, components of the hormone-sensitive AC system were found to be interchangeable with the components of the photoreceptor system, thereby supporting the notion that, despite the wide range of external stimuli, all GPCRs might function via a common mechanism (42).
Visual Signal Transduction Pathway
In contrast to the AC system, the molecular identity of rhodopsin came from studies on the visual pigment itself. Rhodopsin was known by other names in the early days of its research such as visual red, visual purple, and erythropsin (43, 44). The initial detection of rhodopsin came from its reddish-purple color. One of the first to detect this color may have been Heinrich Müller, who in 1851 noted the red color of rod cells (reviewed in refs 43 and 44). Franz Boll was the first to notice the deterioration of this red color by light in 1876 and that the color could be regenerated in the dark. He suggested that this light-dependent redness in the retina might derive from either a photochemical or photophysical process. If it derived from the former, he suggested that the red color might be a pigment analogous to hemoglobin in red blood cells. At about the same time, Kühne demonstrated that the effect of light on the reddish-purple color was of a photochemical nature, and he was the first to extract this pigment using bile salts. Hecht suggested that vision proceeded via a bimolecular process where the visual pigment is reversibly broken down into two components (45, 46). Large amounts of vitamin A were found in the retina by Wald (47), and this led to the idea of retinoids as a potential source of the visual pigment. His observations were also in apparent agreement with a bimolecular structure for the pigment, and he suggested that the pigment was a protein conjugated to a retinoid (48).
Molecular Characterization of GPCRs
The molecular characterization of rhodopsin preceded that of any other GPCR. This progress was mainly due to the high concentration and purity in which rhodopsin is found in rod outer segments, where the photopigment represents ∼70% of the total protein in the cell (49). Rhodopsin was the first GPCR to have its full amino acid sequence deciphered (50, 51) and is the first, and currently only, GPCR for which a high-resolution crystal structure is available (52). Despite the wealth of information accumulated for this receptor, its oligomeric status remains a subject of some discussion (53).
Rod outer segments are composed of stacked disc membranes that are enclosed by a plasma membrane (54, 55). Rhodopsin is found in both the plasma membrane and the disc membranes. Only the form found in the disc membranes is known for certain to participate in signal transduction. Rhodopsin also appears to play a role in the morphology of the rod outer segments (56). Early X-ray diffraction studies indicated that rhodopsin is dispersed throughout the rod outer segment disk membranes in a planar liquid-like arrangement (57, 58). Freeze fracture electron microscopy also supported the dispersed and densely packed nonordered arrangement of rhodopsin within the membrane (59, 60). It is unclear whether the detected species correspond to a monomeric or oligomeric form of rhodopsin since the measured diameters using these techniques point to dimers or larger oligomers of rhodopsin (59-61).
The notion that rhodopsin occurs as monomers had come in part from photobleaching and transient dichroism measurements which reveal rapid lateral and rotational diffusion constants associated with the protein (62-65). The rapid mobility of rhodopsin was indicative of a protein that freely moves through the membrane, a notion inconsistent with large oligomeric complexes. This observation was supported by the observation that glutaraldehyde fixation of rhodopsin slows the diffusional rate of the protein (66). The behavior of rhodopsin in the membrane appeared to be consistent with the traditional fluid mosaic model of cell membranes (67), and this concept led in part to the widespread acceptance that the pigment was monomeric. Such a model for cell membrane dynamics is now known to be inaccurate. Microdomains and lipid rafts are known to exist within the membrane, and these locations in the cell membrane have been shown to house components of GPCR signal transduction (68-71). In addition, it is unclear whether the measured diffusional rates can be applied to all rhodopsin in rod outer segments. It has been determined that approximately a quarter of rhodopsin molecules are immobile and that the mobility of rhodopsin differs depending on the axial location of the disks in which they are found (65).
The notion that rhodopsin freely diffuses through the membrane has led to the disregard of suggestions that rhodopsin may form oligomers. For instance, chemically cross-linked rhodopsin oligomers have been detected with various cross-linkers (72-74); however, those species were speculated to derive from random collision due to the high density of rhodopsin found in the disk membranes. The appearance of various oligomeric species over time was measured and compared with theoretical time courses. While this ruled out the possibility of dimers, it did not necessarily rule out tetramers or larger oligomers (72).
Among early reports about the molecular characterization of other GPCRs, direct and convincing evidence for oligomers was largely absent. The size of various GPCRs had been largely estimated from their mobility during gel filtration, ultracentrifugation, and gel electrophoresis. Monomers of GPCRs were readily detected in these types of assays. The detection of oligomers, on the other hand, was more variable. In some cases, they represented only a minor fraction of the total receptor population, while in other cases, they represented an appreciable fraction (75-78). In studies on unprocessed extracts or partially purified material, aggregates identified under nondenaturing conditions likely contained other constituent proteins such as the G protein.
A prerequisite for biochemical studies is extraction of the membrane-bound receptor into detergent, which leads to an extrapolation from its status during the assay to that in the native membrane. The preservation of the native state of GPCRs in detergent is not a trivial task. Aggregation of GPCRs can occur in a manner that may not be possible under physiological conditions. For instance, heating soluble extracts of the M2 muscarinic receptor has been shown to promote aggregation of the receptor (79). Also, the crystal structure of rhodopsin in nonyl β-glucoside has revealed a dimer that is in a nonphysiological orientation (80). Detergents can also have the opposite effect whereby preformed oligomers disassemble into monomers upon solubilization. For instance, aggregates of rhodopsin have been shown to be differentially preserved depending on the detergent used for extraction (ref 211).2
In addition to the potential effects of detergents on the oligomeric status of receptors are the potential effects of detergents on the activity of receptors. Assays dependent on the activity of receptors will be biased if the detergent affects one population of receptors differently than another. For example, solubilized extracts from Sf9 cells coexpressing different tagged forms of the M2 muscarinic receptor have been shown to contain a mixture of monomers and oligomers (79). Only the monomeric form of the receptor was able to bind antagonists, an effect attributed in part to the detergent. In such a scenario, only the monomers would be detected in hydrodynamic studies where the mobility of the receptor is monitored by radiolabeled ligands. The potential effects of detergents and the nature of the biochemical assays themselves complicate the interpretation of the data and in some instances may have biased the results in favor of monomers. Such complications may have contributed to an under-appreciation of GPCR oligomerization.
Radiation inactivation studies have been performed to avoid the complications of solubilization and to gain insight into the status of GPCRs in their native environment. Such studies have been performed on membranes containing various GPCRs, including the muscarinic, adrenergic, and dopamine receptors (81-88), and in several cases, the estimated molecular weight corresponds to a dimer. There are many assumptions underlying such inferences, and therefore, the interpretation of the data is not straightforward (89). The technique is further complicated by the fact that GPCRs are in complex with other proteins within the membrane. Although these studies point to an oligomeric organization of GPCRs within the membrane, they may not necessarily represent the true native oligomeric status of GPCRs.
Recent Evidence for Oligomers of GPCRs
More recent biochemical and biophysical studies have provided a more direct and less ambiguous demonstration that GPCRs can exist as oligomers. Most of those studies have involved measurements of co-immunoprecipitation and resonance energy transfer. Results from recent studies demonstrating the oligomeric nature of GPCRs have been covered in several reviews (90-94). Co-immunoprecipitation has demonstrated directly that oligomers can exist, and it avoids the uncertainties associated with bands that migrate as oligomers during gel electrophoresis. Controls have been conducted to demonstrate that the detection of oligomers by co-immunoprecipitation is not an artifact of the solubilization conditions (95, 96). The detection of GPCR oligomers on the surface of intact cells has been facilitated by the detection of resonance energy transfer between receptors labeled with bioluminescent or fluorescent probes. Both approaches indicate that most if not all GPCRs can exist as homo-oligomers (92, 94, 97-99).
More recently, atomic force microscopy of native disk membranes from rod outer segments has revealed that rhodopsin is arranged in large paracrystalline arrays, providing perhaps the clearest demonstration of GPCR oligomerization (100). While this suggests the possibility that GPCRs form large arrays, it is unclear whether this is the functional arrangement of rhodopsin. These results are in contrast with the earlier studies mentioned above. Nonetheless, these studies visually demonstrate the propensity of rhodopsin to form oligomers under native conditions.
Although the dimerization of GPCRs is now a widely accepted idea and has been studied extensively, the possibility that GPCRs form higher-order oligomers has received less attention. The ligand-binding properties of cardiac muscarinic receptors point to at least four interacting sites when noncompetitive effects and the heterogeneity revealed by agonists are described in terms of cooperative interactions (101-103). Other indirect estimates of oligomeric size have been inferred from resonance energy transfer, co-immunoprecipitation, and disulfide trapping data (95, 104-106). The conclusions are somewhat variable, as some of the data point to dimers while others point to larger oligomers. Direct evidence for an oligomer as large as a trimer has come from the copurification of three differently tagged forms of the M2 muscarinic receptor coexpressed in Sf9 cells (107). Statistical considerations in that study point to an oligomer that may be even larger.
Several lines of evidence support the idea that oligomers of GPCRs are formed prior to being transported to the cell surface (reviewed in ref 108). For example, mutants of the β2 adrenergic receptor (109), the D2 dopamine receptor (110), the platelet-activating factor receptor (111), and the V2 vasopressin receptor (112) can inhibit the transport of the respective wild-type receptors to the cell surface. Likewise, the disruption of oligomerization by mutating the putative dimerization motif of the β2 adrenergic receptor and the α-factor receptor has been shown to hinder the cell surface expression of both receptors (109, 113). In addition, bioluminescence resonance energy transfer between tagged oxytocin receptors, vasopressin receptors (114), and β2 adrenergic receptors (109) has been detected in membrane fractions enriched in endoplasmic reticulum.
It is unclear at the moment whether oligomerization during biogenesis is a general pathway for all GPCRs. Also unclear is the stability of those preformed oligomers during signaling. Oligomerization of some GPCRs has been reported to occur at the cell surface in a process regulated by ligands (reviewed in refs 99 and 108). Ligands have also been reported to affect the oligomeric integrity of preformed aggregates of some GPCRs. No consensus is yet available on these effects, which may reflect the ambiguity associated with the interpretation of the data (108). Alternatively, the lack of consistency may reflect differences in the role of oligomers in signaling for different GPCRs. For instance, preformed oligomers of some GPCRs may serve as a signaling platform that retains its multimeric status throughout the process. Other GPCRs may cycle through monomeric and multimeric states in a ligand-regulated process that itself lies in the signaling pathway.
Various regions of GPCRs are thought to be involved in forming the interface between monomeric units (reviewed in ref 115). These regions have been inferred from studies involving chemical cross-linking, site-directed mutagenesis, computational analyses, molecular modeling, and coexpression of receptor fragments (100, 106, 109, 113, 115-120). The transmembrane domains that are thought to be involved at the oligomerization interface include transmembrane helices 1 and 4–6. Also thought to be involved in oligomerization are the cytoplasmic loops formed between transmembrane helices 1 and 2 and transmembrane helices 5 and 6 (120).
Hetero-oligomerization between different GPCRs has been demonstrated in similar biochemical and biophysical studies with a variety of different receptors and subtypes (reviewed in refs 93 and 121). Hetero-oligomers can have distinct properties that demonstrate the functional potential of GPCR oligomers. For instance, membranes from COS-7 cells transfected with the genes for two complementary chimeras of the α2 adrenergic and M3 muscarinic receptors were found to bind adrenergic or muscarinic antagonists only when the recombinant proteins were coexpressed, thereby implying an association between the two chimeras. The coexpressed chimeras were also able to mediate the hydrolysis of phosphatidylinositol (122, 123). Heteromeric somatostatin receptors have been implicated in the inhibition of AC, internalization, and upregulation (124). Similarly, enhanced functional activity has been reported for the hetero-oligomers formed by angiotensin AT1 and bradykinin receptors (125), by the D2 dopamine and SST-5 somatostatin receptors (126), and by the μ and δ opioid receptors (127). Perhaps the best example demonstrating the functional importance of oligomerization is given by the γ-aminobutyric acid (GABAB) receptor, which exists as a heteromer of GABAB1 and GABAB2 subunits. Oligomerization is a prerequisite for the trafficking of the hetero-oligomer to the cell surface, and the allosteric interactions between the subunits of the GABAB receptor are critical for agonist-induced activation (128-132).
Reports on the oligomerization of GPCRs continue to accumulate at a rapid pace. Oligomers occur across all families of GPCRs, and they have been implicated in many aspects of signaling, including trafficking, desensitization, signaling per se, and pharmacological diversity (99, 101, 108). In a reversal of conventional wisdom, this prevalence of oligomers calls into question the role and even the existence of monomers in nature. Preparations of rhodopsin and the M2 muscarinic receptor known to comprise predominantly monomers have been prepared (79, 211). In the case of the M2 muscarinic receptor, monomers appear to possess at least some of the functionality displayed by the native receptor since they bind antagonists with characteristic muscarinic affinity, but the relevance of such forms to signaling remains unclear.
Mechanism of Signal Transduction
The classical view of G protein-mediated signaling presumes that signaling proceeds via a monomeric receptor coupled transiently to its G protein in a 1:1 stoichiometry (133-135). This view has emerged in part from studies on the turnover of the G protein, from biochemical studies on the interaction between the receptor and G protein, and from the interpretation of the complex binding patterns revealed in the binding of agonists to the receptor. The latter studies have led to the ternary complex model, and this mechanistic and conceptual framework is still the most prevalent one used today (136, 137). Many GPCRs exhibit an apparent heterogeneity in the binding of agonists to the receptor (i.e., nH < 1) and a guanyl nucleotide-regulated interconversion between the different states (101, 133, 138-142). Those properties are not predicted by classical pharmacological models (e.g., Figure 2, Schemes 1 and 2; 8-10). The importance of such effects on signaling is suggested by the correlation between measures of the magnitude of the guanyl nucleotide-modulated dispersion and indicators of an agonist's physiological effectiveness such as efficacy and intrinsic activity (142-146), thereby implying a central role of those effects in G protein-mediated signaling. In addition, changes in those patterns are sometimes associated with disease (147-149).
The number of possible explanations for a dispersion of affinities in a system at thermodynamic equilibrium is limited, and three possibilities have been examined in some detail: multiple classes of functionally distinct, noninterconverting, and mutually independent sites (i.e., Figure 2, Scheme 3, multisite model), interaction of the receptor with a limiting quantity of a third component such as a G protein (i.e., Figure 2, Scheme 4, ternary complex model), and cooperativity among interacting sites (i.e., Figure 2, Scheme 5). The properties and merits of these mechanistic schemes have been discussed previously (101, 138, 150-153).
Multisite Model and Ternary Complex Model
The disperse or “flat” curves revealed by agonists are most often analyzed quantitatively according to the multisite model (154). In such a scheme, the dispersion derives from multiple classes of mutually independent and noninterconverting receptors with distinct affinities for the agonist. The data can often be described in terms of two classes, one with a high affinity (KA1) and another with a weaker affinity (KA2) for the agonist. While such a scheme is consistent with data obtained with a single agonist, it is inconsistent with the data from multiple agonists taken together (138, 143, 155). Moreover, the guanyl nucleotide-dependent interconversion between different states for a single agonist cannot be accommodated by such a model.
Although the multisite model fails as an adequate mechanistic model to describe G protein-mediated signaling, it is often used in conjunction with the assumed premise of the ternary complex model to rationalize the binding behavior of agonists (141-143). The data are often quantitatively analyzed in terms of the multisite model while qualitatively discussed in terms of components in the ternary complex model. Such a rationalization must be approached with caution since there is no direct relationship between the two models, and estimates of affinity from the former (i.e., KA1 and KA2) are therefore not necessarily equal to the affinities from the latter (i.e., KAG and KA) (139, 142, 151).
The heterogeneity revealed by agonists is qualitatively rationalized in terms of a difference in the affinity of the agonist for the receptor coupled to the G protein (RG, KAG) and the uncoupled receptor (R, KA) in a manner that is analogous to the high- and low-affinity sites of the multisite model (i.e., KA1 and KA2). The effect of the guanyl nucleotide is to shift the equilibrium to the uncoupled state, and therefore, the affinity of the agonist will be weaker in the presence of guanyl nucleotides, resulting in a rightward shift (“GTP shift”). The breadth of the GTP shift is often used as an indicator of the efficiency in coupling of the receptor to its G protein, and conditions under which this effect is absent are interpreted as an inability of the receptor to form an RG complex. The ternary complex model does not support such a qualitative description, however, when applied quantitatively to agonist binding data (138, 139, 150, 155). The nature of such a scheme limits the quantitative description of the data to specific conditions that may not occur physiologically (151, 153, 156). Moreover, parameters computed from the point of view of the receptor are inconsistent with the same parameters derived from the point of view of the G protein (150).
Cooperativity, Oligomerization, and Signaling
An alternative to the ternary complex model is a scheme based on the notion of cooperativity. Since there appears to be one orthosteric binding site per receptor molecule (157, 158), cooperativity implies that the receptor is oligomeric. This is consistent with our current knowledge of receptor structure and suggests that the oligomers have a mechanistic role in signaling. Heterogeneity has been observed in the binding of agonists to the affinity-purified M2 muscarinic receptor devoid of G proteins, which points to an effect that is intrinsic to the receptor itself (102, 159). Cooperativity within a tetravalent receptor has been shown to provide a quantitatively consistent description of the characteristic nucleotide-modulated heterogeneity revealed by agonists at muscarinic receptors in hamster myocardial membranes (Figure 2, Scheme 5) (101).
Cooperativity or apparent cooperativity was detected early in the binding of ligands to GPCRs (152, 160-168). One of the earliest suggestions of cooperativity came from studies on β-adrenergic receptors from frog erythrocytes, where low Hill coefficients observed in the binding of [3H]alprenolol were rationalized in terms of negative cooperativity between interacting sites (162). More recently, negative cooperativity has been observed in the binding of antagonists to the M2 muscarinic receptor and D2 dopamine receptor (103, 169). In each case, one antagonist appeared to inhibit another in a noncompetitive manner. The data were consistent with cooperativity among two or four interacting sites for the D2 dopamine receptor and M2 muscarinic receptor, respectively. Cooperativity has also been detected within hetero-oligomeric complexes where subtype- or receptor-specific ligands for one type or subtype of receptor affect the binding of those specific for another (126, 127, 170, 171).
Scheme 5 (Figure 2) describes the binding of a ligand (agonist, A) to a tetravalent receptor that interconverts spontaneously between two states (receptor in the R and T state) (101). Such a scheme is based on the combined approaches of Koshland et al. and Monod et al. (172, 173). Each site or monomer is functionally linked, and therefore, the binding of 1 equiv of agonist may potentially affect the binding of subsequent equivalents of the ligand. The detection of constitutive activity and its regulation by ligands have indicated that most if not all GPCRs can exist in at least two states: an active state favored by agonists and an inactive state favored by inverse agonists (174-179). Both states appear to be intrinsic to the receptor itself, since they are observed in affinity-purified cardiac muscarinic receptors devoid of G proteins (180). The same ligand-regulated equilibrium between two states also is likely to account for the effect of guanyl nucleotides and the G protein on the binding of agonists as described above. The T state may represent the active receptor that elicits a biological response, and the interconversion between the R and T states may be regulated by agonist, inverse agonist, and guanyl nucleotides. The state of coupling between the receptor and the G protein is not explicitly represented, and therefore, R and T may represent either a stable or transient receptor–G protein pair. If Scheme 5 or a similar model were to hold up under further testing, it would suggest that cooperative interactions between sites of an oligomer are critical for, and directly result in, the propagation of an external signal.
Oligomeric Structure of GPCRs Implied by Other Signaling Components
The size of various signaling components and the predicted GPCR contact sites on those proteins are also consistent with the notion of an oligomeric GPCR (Figures 3 and 4). Structural information about the components of G protein-mediated signaling is most abundant for members of the visual signal transduction cascade (181). Results from the molecular modeling of some of those components point to an oligomeric arrangement of rhodopsin (54, 100). Arrestin is a protein that binds to phosphorylated GPCRs and is involved in the desensitization of the activated receptor, and more recently has been shown to link GPCRs to G protein-independent signaling pathways (182). The footprint on visual arrestin for rhodopsin is suggestive of oligomerization (100). Two grooves separated by 3.8 nm are found within the structure of arrestin, which can accommodate two molecules of phosphorylated rhodopsin (Figure 3).
Figure 3.
Ribbon and space-filling model of a complex between a rhodopsin dimer and a monomer of arrestin. Arrestin (purple) has a bipartite structure that can accommodate two molecules of rhodopsin (yellow). Phosphorylation sites on rhodopsin are represented by green spheres (adapted from ref 100).
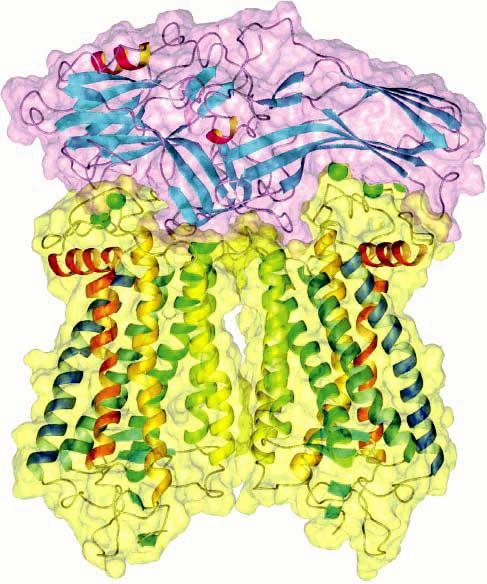
Figure 4.

Ribbon and space-filling model of a complex between two rhodopsin dimers and a dimer of Gtα. Information obtained from atomic force microscopy and from the crystal structures of rhodopsin and transducin suggests a 2:1 stoichiometry between rhodopsin (blue) and Gtα (orange). Signaling may proceed via oligomers of rhodopsin and oligomers of Gtα (adapted from ref 54).
The photoreceptor heterotrimeric G protein transducin, Gt, is larger than a monomeric rhodopsin. In fact, if it is assumed that rhodopsin forms dimers, it has been shown that extensive contacts are made between the α subunit of transducin (Gtα) and dimeric rhodopsin (Figure 4) (54, 100). In such a scenario, the β subunit of Gt makes contact with four monomers of rhodopsin and is required to dissociate prior to the binding of further equivalents of Gtα (Supporting Information). This sizing is suggestive of a 2:1 ratio between receptor and Gtα, which differs from the conventional view of a 1:1 stoichiometry between the two signaling molecules. The 2:1 stoichiometry is also supported by the detection of a pentameric assembly composed of a heterotrimeric G protein and dimeric leukotriene B4 receptor (183).
Molecular simulations have also indicated that Gtα bound to adjacent rhodopsin dimers can be in contact with each other (Figure 4) (54). This suggests that Gtα may form oligomeric arrays similar to the arrays formed by the receptor and that the binding of one Gtα may facilitate the binding of the next equivalent of Gtα. Indeed, apparent Hill coefficients of ∼2 have been observed in the binding of Gt to activated rhodopsin (184, 185). Moreover, the maximal binding capacity for Gt represented only 25% of activated rhodopsin. This discrepancy in binding capacity was due neither to a kinetic artifact nor to accessibility issues (185), and it thereby indirectly indicated that Gt may bind to an oligomeric rhodopsin (184, 186).
Positive cooperativity is observed in the binding of guanyl nucleotides to G proteins in hamster cardiac membranes (187), where the muscarinic receptor agonist carbachol promotes a bell-shaped pattern in the effect of GDP on bound [35S]GTPγS under some conditions. Moreover, heterogeneity is observed in the binding of guanyl nucleotides to the G protein and is modulated by agonists in a manner that mirrors the guanyl nucleotide-modulated heterogeneity observed in the binding of agonists (150, 187-189). Taken together, these data are suggestive of a symmetrical system wherein effects within the oligomeric array of the receptor are mirrored by the effects that occur within the oligomeric array of the G protein. This apparent symmetry cannot be accommodated within the context of the ternary complex model (150).
Several lines of evidence support the notion that the heterotrimeric G protein, or its α subunit, can form oligomeric clusters (190-197). For example, dimeric forms of both the α subunit and the βγ complex of Gt, as well as tetrameric forms of the latter, have been detected on native gels and by analytical ultracentrifugation (190). Radiation inactivation of the glucagon receptor implied molecular weights corresponding to multiples of the receptor and the G protein (191). Chemical cross-linking studies have indicated that Gt,Gαs,Gαi,Gαo, and Gβ can all exist in oligomeric arrays (192, 193). A dimeric organization of Gαi1 is detected in its crystal structure (194); however, the physiological relevance of that complex is unknown.
Structural Basis of GPCR Activation
Structural information about GPCRs is limited, and therefore, a structural understanding of receptor activation is still in the early stages. Activation of GPCRs has been shown to result in a rearrangement of the transmembrane helices (198, 199). Recent studies on metabotropic glutamate receptors (mGluRs) demonstrate how those rearrangements might affect the oligomeric structure and function of the receptor (200-202). Fluorescence resonance energy transfer measurements made on modified mGluR1α suggest that the first intracellular loops of each monomer move apart while the second intracellular loops move closer together upon activation by an agonist (201). Functional studies on chimeric mGluR5 indicate that the binding of an agonist to the amino-terminal domain of one subunit can produce a response by activating the transmembrane domain of another subunit (202).
A more complete structural picture of GPCR activation may come from studies on rhodopsin. This system is amenable to various biophysical techniques that will allow for the visualization of macromolecules at high resolution. The crystal structures for the active and inactive states of multimeric proteins such as hemoglobin and aspartate transcarbamoylase have played a large part in understanding their function (203). The high-resolution crystal structure is currently available only for the inactive state of rhodopsin (52) and low-resolution inactive intermediate Meta I (204). The availability of a high-resolution crystal structure of the active form of rhodopsin may be required to gain similar insights into the activation and function of GPCRs. A major drawback of crystal structures, however, is that they do not allow for the study of membrane-bound proteins in their natural environment. The requirement for extraction and the crystallization processes most likely alters the structure of rhodopsin to some extent, especially its oligomeric structure. Information from crystal structures will therefore need to be combined with techniques allowing for the visualization of the protein under more native conditions.
Atomic force microscopy allows for the high-resolution detection of proteins in their natural lipid environment (205). This technique has already provided images of rhodopsin in native disk membranes of rod outer segments (100). Those images were not sufficiently detailed to distinguish the loops connecting the transmembrane helices of rhodopsin like those observed in studies with bacteriorhodopsin (206). Higher-resolution images that can detect the finer details of the disk membrane topography will allow for a more accurate representation of the orientation of rhodopsin in the membrane that is independent of molecular modeling (100, 120). The ability to obtain such images may allow for the visualization of the structural changes that occur upon activation within the context of the native membrane environment. High-resolution atomic force microscopy can also be combined with single-molecule force spectroscopy (207). This technique allows for the manipulation of individual proteins and provides information about the inter- and intramolecular interactions of that protein. Activation of the receptor will alter those interactions, and studies of this nature may be able to detect those changes.
Advances in cryoelectron tomography have allowed for visualization of the macromolecular architecture of whole cells and organelles at a resolution of 4–5 nm (208). This resolution limits the detection to only large protein complexes (>400 kDa); however, advances may make higher-resolution detection possible soon. The diameter of mouse rod outer segments is ∼1μm (100). This size makes them suitable for cryoelectron tomography without the need for sectioning, making the technique relatively noninvasive. Also, the relatively simple composition of rod outer segments may facilitate the imaging and recognition of its components. Such an approach will therefore allow one to view a snapshot of rhodopsin not only in its natural membrane but also within the context of its natural compartment.
Concluding Remarks
The notion that GPCRs are monomeric and that signaling occurs via a transient 1:1 complex of the receptor and G protein has predominated for much of the field's history. More recent evidence, however, points to an oligomeric arrangement of GPCRs and highlights a need to modify our current conceptualization of G protein-mediated signaling. Despite the attention that the notion of GPCR oligomerization has been receiving, data are still largely interpreted within the framework of the traditional view of signaling. An alternate mechanistic framework based on the notion of cooperativity among interacting sites of an oligomeric receptor has been considered here. Such a framework may more accurately represent the system and therefore lead to a better understanding of function and dysfunction in GPCRs.
Cooperative schemes were initially proposed to describe the allosteric behavior of hemoglobin and multisubunit enzymes such as aspartate transcarbamoylase (reviewed in ref 203). Cooperativity is critical to the function of those oligomeric proteins and is linked to changes in their quaternary structure. In addition, Wyman pointed out that the potential fineness of allosteric control of a macromolecule increases with the number of interacting sites (209). The organization of GPCRs into complexes containing multiple equivalents of both receptor and G protein provides a platform for various cooperative effects and may allow for a form of fine control in GPCR-mediated signaling. GPCRs represent the initial stage in a cascade of events leading to a biological response. These signaling cascades are seemingly complex and are often critical to the vitality of an organism. It is expected then that precise controls would be integrated into the system to ensure the orderly function of a seemingly complex situation.
ACKNOWLEDGMENT
We thank Dr. Lakshmi A. Devi, Dr. Jonathan A. Javitch, Dr. Brian K. Kobilka, Dr. Graeme Milligan, and Dr. Kevin D. Ridge for helpful comments on the manuscript.
Footnotes
This work was supported in part by U.S. Public Health Service Grant EY08061 from the National Eye Institute (National Institutes of Health, Bethesda, MD), an unrestricted grant from Research to Prevent Blindness, Inc. (RPB, New York, NY), to the Department of Ophthalmology at the University of Washington, and a grant from the E. K. Bishop Foundation to K.P. This study also was supported by funds from Polish State Committee for Scientific Research (Grant 3P05F02625 to S.F.), the Canadian Institutes of Health Research (Grant MOP43990, to J.W.W.), and the Heart and Stroke Foundation of Ontario (Grant T4914, to J.W.W.). P.S.-H.P. is the recipient of a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada.
Abbreviations: A, agonist; AC, adenylate cyclase; ACTH, adrenocorticotropic hormone; G, G protein; Gα, α subunit of the G protein; Gβ, β subunit of the G protein; Gγ, γ subunit of the G protein; Gt, photoreceptor G protein, transducin; GABA, γ-aminobutyric acid; GPCR, G protein-coupled receptor; mGluR, metabotropic glutamate receptor; R, receptor; RG, receptor–G protein complex; TSH, thyroid-stimulating hormone.
Also, K. Suda, S. Filipek, A. Engel, and D. Fotiadis, unpublished results.
SUPPORTING INFORMATION AVAILABLE An animated video illustrating the potential interaction between rhodopsin (ground-state, blue; photon-activated, yellow) and transducin (Gα, orange; Gβ, red; Gγ, green). GTP is represented in purple, and GDP is represented in light purple. The video is based on information obtained from atomic force microscopy and molecular modeling using information from the crystal structures of the two components (54, 100, 120). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Takeda S, Kadowaki S, Haga T, Takaesu H, Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 2.Ma P, Zemmel R. Value of novelty? Nat. Rev. Drug Discovery. 2002;1:571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 3.Wise A, Gearing K, Rees S. Target validation of G-protein coupled receptors. Drug Discovery Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- 4.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: An evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada T, Ernst OP, Palczewski K, Hofmann KP. Activation of rhodopsin: New insights from structural and biochemical studies. Trends Biochem. Sci. 2001;26:318–324. doi: 10.1016/s0968-0004(01)01799-6. [DOI] [PubMed] [Google Scholar]
- 6.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 8.Clark AJ. The mode of action of drugs on cells. Edward Arnold & Co.; London: 1933. [Google Scholar]
- 9.Furchgott RF. The use of β-haloalkylamines in the differentiation of receptors and in the determination of dissociation constants of receptor-agonist complexes. Adv. Drug Res. 1966;3:21–55. [Google Scholar]
- 10.Stephenson RP. A modification of receptor theory. Br. J. Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrlich P. Address in pathology on chemotherapeutics: Scientific principles, methods, and results. Lancet. 1913;182:445–451. [Google Scholar]
- 12.Langley JN. On the physiology of the salivary secretion. J. Physiol. 1878;1:339–369. doi: 10.1113/jphysiol.1878.sp000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley JN. On the reaction of cells and of nerve-endings to certain poisions, chiefly as regards the reaction of striated muscle to nicotine and to curari. J. Physiol. 1905;33:374–413. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dale HH. The action of certain esters and ethers of choline, and their relation to muscarine. J. Pharmacol. Exp. Ther. 1914;6:147–190. [Google Scholar]
- 15.Langmuir I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916;38:2221–2295. [Google Scholar]
- 16.Ariens EJ. Affinity and intrinsic activity in the theory of competitive inhibition. I. Problems and theory. Arch. Int. Pharmacodyn. Ther. 1954;99:32–49. [PubMed] [Google Scholar]
- 17.Ariens EJ, van Rossum JM, Simonis AM. Affinity, intrinsic activity and drug interactions. Pharmacol. Rev. 1957;9:218–236. [PubMed] [Google Scholar]
- 18.Black JW, Leff P. Operational models of pharmacological agonism. Proc. R. Soc. London, Ser. B. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland EW, Rall TW, Menon T. Adenyl cylase. I. Distribution, preparation, and properties. J. Biol. Chem. 1962;237:1220–1227. [PubMed] [Google Scholar]
- 20.Sutherland EW, Cori CF. Effect of hyperglycemicglycogenolytic factor and epinephrine on liver phosphorylase. J. Biol. Chem. 1951;188:531–543. [PubMed] [Google Scholar]
- 21.Rall TW, Sutherland EW, Berthet J. The relationship of epinephrine and glucagon to liver phosphorylase. IV. Effect of epinephrine and glucagon on the reactivation of phosphorylase in liver homogenates. J. Biol. Chem. 1957;224:463–475. [PubMed] [Google Scholar]
- 22.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J. Biol. Chem. 1958;232:1077–1091. [PubMed] [Google Scholar]
- 23.Haynes RC., Jr. The activation of adrenal phosphorylase by the adrenocorticotropic hormone. J. Biol. Chem. 1958;233:1220–1222. [PubMed] [Google Scholar]
- 24.Klainer LM, Chi YM, Freidberg SL, Rall TW, Sutherland EW. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3′,5′-phosphate by preparations from brain and other tissues. J. Biol. Chem. 1962;237:1239–1243. [PubMed] [Google Scholar]
- 25.Mansour TE, Sutherland EW, Rall TW, Bueding E. The effect of serotonin (5-hydroxytryptamine) on the formation of adenosine 3′,5′-phosphate by tissue particles from the liver fluke, Fasciola hepatica. J. Biol. Chem. 1960;235:466–470. [PubMed] [Google Scholar]
- 26.Murad F, Chi YM, Rall TW, Sutherland EW. Adenyl cyclase. III. The effect of catecholamines and choline esters on the formation of adenosine 3′,5′-phosphate by preparations from cardiac muscle and liver. J. Biol. Chem. 1962;237:1233–1238. [PubMed] [Google Scholar]
- 27.Robison GA, Butcher RW, Sutherland EW. Adenyl cyclase as an adrenergic receptor. Ann. N.Y. Acad. Sci. 1967;139:703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- 28.Birnbaumer L, Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J. Biol. Chem. 1969;244:3477–3482. [PubMed] [Google Scholar]
- 29.Butcher RW, Baird CE, Sutherland EW. Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J. Biol. Chem. 1968;243:1705–1712. [PubMed] [Google Scholar]
- 30.Paton WDM, Rang HP. The uptake of atropine and related drugs by intestinal smooth muscle of the guinea-pig in relation to acetylcholine receptors. Proc. R. Soc. London, Ser. B. 1965;163:1–44. doi: 10.1098/rspb.1965.0058. [DOI] [PubMed] [Google Scholar]
- 31.Lefkowitz RJ, Roth J, Pricer W, Pastan I. ACTH receptors in the adrenal: Specific binding of ACTH-125I and its relation to adenyl cyclase. Proc. Natl. Acad. Sci. U.S.A. 1970;65:745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodbell M, Krans HM, Pohl SL, Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: Method of assay and specificity. J. Biol. Chem. 1971;246:1861–1871. [PubMed] [Google Scholar]
- 33.Haga T, Haga K, Gilman AG. Hydrodynamic properties of the β-adrenergic receptor and adenylate cyclase from wild type and varient S49 lymphoma cells. J. Biol. Chem. 1977;252:5776–5782. [PubMed] [Google Scholar]
- 34.Limbird LE, Lefkowitz RJ. Resolution of β-adrenergic receptor binding and adenylate cyclase activity by gel exclusion chromatography. J. Biol. Chem. 1977;252:799–802. [PubMed] [Google Scholar]
- 35.Welton AF, Lad PM, Newby AC, Yamamura H, Nicosia S, Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J. Biol. Chem. 1977;252:5947–5950. [PubMed] [Google Scholar]
- 36.Orly J, Schramm M. Coupling of catecholamine receptor from one cell with adenylate cyclase from another cell by cell fusion. Proc. Natl. Acad. Sci. U.S.A. 1976;73:4410–4414. doi: 10.1073/pnas.73.12.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birnbaumer L. Transduction of receptor signal into modulation of effector activity by G proteins: The first 20 years or so. FASEB J. 1990;4:3178–3188. doi: 10.1096/fasebj.4.14.2172060. [DOI] [PubMed] [Google Scholar]
- 38.Rodbell M, Birnbaumer L, Pohl SL, Krans HM. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J. Biol. Chem. 1971;246:1877–1882. [PubMed] [Google Scholar]
- 39.Rodbell M, Krans HM, Pohl SL, Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J. Biol. Chem. 1971;246:1872–1876. [PubMed] [Google Scholar]
- 40.Ross EM, Gilman AG. Resolution of some components of adenylate cyclase necessary for catalytic activity. J. Biol. Chem. 1977;252:6966–6969. [PubMed] [Google Scholar]
- 41.Wheeler GL, Bitensky MW. A light-activated GTPase in vertebrate photoreceptors: Regulation of light-activated cyclic GMP phosphodiesterase. Proc. Natl. Acad. Sci. U.S.A. 1977;74:4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bitensky MW, Wheeler MA, Rasenick MM, Yamazaki A, Stein PJ, Halliday KR, Wheeler GL. Functional exchange of components between light-activated photoreceptor phosphodiesterase and hormone-activated adenylate cyclase systems. Proc. Natl. Acad. Sci. U.S.A. 1982;79:3408–3412. doi: 10.1073/pnas.79.11.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boll F. On the anatomy and physiology of the retina. Vision Res. 1977;17:1249–1265. doi: 10.1016/0042-6989(77)90112-2. [DOI] [PubMed] [Google Scholar]
- 44.Kuhne W. Chemical processes in the retina. Vision Res. 1977;17:1269–1316. doi: 10.1016/0042-6989(77)90114-6. [DOI] [PubMed] [Google Scholar]
- 45.Hecht S. The photochemical nature of the photosensory process. J. Gen. Physiol. 1920;2:229–246. doi: 10.1085/jgp.2.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hecht S. The dark adaptation of the human eye. J. Gen. Physiol. 1920;2:499–517. doi: 10.1085/jgp.2.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wald G. Vitamin A in the eye tissues. J. Gen. Physiol. 1935;18:905–915. doi: 10.1085/jgp.18.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wald G. Carotenoids and the visual cycle. J. Gen. Physiol. 1935;19:351–371. doi: 10.1085/jgp.19.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamm HE, Bownds MD. Protein complement of rod outer segments of frog retina. Biochemistry. 1986;25:4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- 50.Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, Fong SL, Rao JK, Argos P. The structure of bovine rhodopsin. Biophys. Struct. Mech. 1983;9:235–244. doi: 10.1007/BF00535659. [DOI] [PubMed] [Google Scholar]
- 51.Ovchinnikov Yu. A. Rhodopsin and bacteriorhodopsin: Structure–function relationships. FEBS Lett. 1982;148:179–191. doi: 10.1016/0014-5793(82)80805-3. [DOI] [PubMed] [Google Scholar]
- 52.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le TI, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 53.Chabre M, Cone R, Saibil H. Biophysics: Is rhodopsin dimeric in native retinal rods? Nature. 2003;426:30–31. doi: 10.1038/426030b. [DOI] [PubMed] [Google Scholar]
- 54.Filipek S, Krzysko KA, Fotiadis D, Liang Y, Saperstein DA, Engel A, Palczewski K. A concept for G protein activation by G protein-coupled receptor dimers: The transducin/rhodopsin interface. Photochem. Photobiol. Sci. 2004;3:628–638. doi: 10.1039/b315661c. [DOI] [PubMed] [Google Scholar]
- 55.Hargrave PA, McDowell JH. Rhodopsin and phototransduction. Int. Rev. Cytol. 1992;137B:49–97. doi: 10.1016/s0074-7696(08)62600-5. [DOI] [PubMed] [Google Scholar]
- 56.Liang Y, Fotiadis D, Maeda T, Maeda A, Modzelewska A, Filipek S, Saperstein DA, Engel A, Palczewski K. Rhodopsin signaling and organization in heterozygote rhodopsin knockout mice. J. Biol. Chem. 2004 doi: 10.1074/jbc.M408362200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blasie JK, Worthington CR. Planar liquid-like arrangement of photopigment molecules in frog retinal receptor disk membranes. J. Mol. Biol. 1969;39:417–439. doi: 10.1016/0022-2836(69)90136-3. [DOI] [PubMed] [Google Scholar]
- 58.Chabre M. X-ray diffraction studies of retinal rods. I. Structure of the disc membrane, effect of illumination. Biochim. Biophys. Acta. 1975;382:322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- 59.Chen YS, Hubbell WL. Temperature- and light-dependent structural changes in rhodopsin-lipid membranes. Exp. Eye Res. 1973;17:517–532. doi: 10.1016/0014-4835(73)90082-1. [DOI] [PubMed] [Google Scholar]
- 60.Roof DJ, Heuser JE. Surfaces of rod photoreceptor disk membranes: Integral membrane components. J. Cell Biol. 1982;95:487–500. doi: 10.1083/jcb.95.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olive J, Recouvreur M. Differentiation of retinal rod disc membranes in mice. Exp. Eye Res. 1977;25:63–74. doi: 10.1016/0014-4835(77)90247-0. [DOI] [PubMed] [Google Scholar]
- 62.Liebman PA, Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974;185:457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- 63.Cone RA. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat. New Biol. 1972;236:39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- 64.Poo M, Cone RA. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974;247:438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- 65.Wey CL, Cone RA, Edidin MA. Lateral diffusion of rhodopsin in photoreceptor cells measured by fluorescence photobleaching and recovery. Biophys. J. 1981;33:225–232. doi: 10.1016/S0006-3495(81)84883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Downer NW, Cone RA. Transient dichroism in photoreceptor membranes indicates that stable oligomers of rhodopsin do not form during excitation. Biophys. J. 1985;47:277–284. doi: 10.1016/S0006-3495(85)83917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 68.Feron O, Smith TW, Michel T, Kelly RA. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J. Biol. Chem. 1997;272:17744–17748. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- 69.Huang C, Hepler JR, Chen LT, Gilman AG, Anderson RG, Mumby SM. Organization of G proteins and adenylyl cyclase at the plasma membrane. Mol. Biol. Cell. 1997;8:2365–2378. doi: 10.1091/mbc.8.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu C, Butz S, Ying Y, Anderson RG. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J. Biol. Chem. 1997;272:3554–3559. doi: 10.1074/jbc.272.6.3554. [DOI] [PubMed] [Google Scholar]
- 71.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there? J. Mol. Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 72.Downer NW. Cross-linking of dark-adapted frog photoreceptor disk membranes. Evidence for monomeric rhodopsin. Biophys. J. 1985;47:285–293. doi: 10.1016/S0006-3495(85)83918-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brett M, Findlay JB. Investigation of the organization of rhodopsin in the sheep photoreceptor membrane by using cross-linking reagents. Biochem. J. 1979;177:215–223. doi: 10.1042/bj1770215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw A, Crain R, Marinetti GV, O'Brien D, Tyminski PN. Light-enhanced cross-linking of rhodopsin in rod outer segment membranes as detected by chemical probes. Biochim. Biophys. Acta. 1980;603:313–321. doi: 10.1016/0005-2736(80)90377-6. [DOI] [PubMed] [Google Scholar]
- 75.Dadi HK, Morris RJ. Muscarinic cholinergic receptor of rat brain. Factors influencing migration in electrophoresis and gel filtration in sodium dodecyl sulphate. Eur. J. Biochem. 1984;144:617–628. doi: 10.1111/j.1432-1033.1984.tb08510.x. [DOI] [PubMed] [Google Scholar]
- 76.Avissar S, Amitai G, Sokolovsky M. Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: Subunit assembly may explain high- and low-affinity agonist states. Proc. Natl. Acad. Sci. U.S.A. 1983;80:156–159. doi: 10.1073/pnas.80.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berrie CP, Birdsall NJM, Haga K, Haga T, Hulme EC. Hydrodynamic properties of muscarinic acetylcholine receptors solubilized from rat forebrain. Br. J. Pharmacol. 1984;82:839–851. doi: 10.1111/j.1476-5381.1984.tb16481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lefkowitz RJ, Haber E, O'Hara D. Identification of the cardiac β-adrenergic receptor protein: Solubilization and purification by affinity chromatography. Proc. Natl. Acad. Sci. U.S.A. 1972;69:2828–2832. doi: 10.1073/pnas.69.10.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park PS-H, Wells JW. Monomers and oligomers of the M2 muscarinic cholinergic receptor purified from Sf9 cells. Biochemistry. 2003;42:12960–12971. doi: 10.1021/bi034491m. [DOI] [PubMed] [Google Scholar]
- 80.Filipek S, Stenkamp RE, Teller DC, Palczewski K. G protein-coupled receptor rhodopsin: A prospectus. Annu. Rev. Physiol. 2003;65:851–879. doi: 10.1146/annurev.physiol.65.092101.142611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fraser CM, Venter JC. The size of the mammalian lung β2-adrenergic receptor as determined by target size analysis and immunoaffinity chromatography. Biochem. Biophys. Res. Commun. 1982;109:21–29. doi: 10.1016/0006-291x(82)91560-1. [DOI] [PubMed] [Google Scholar]
- 82.Lilly L, Fraser CM, Jung CY, Seeman P, Venter JC. Molecular size of the canine and human brain D2 dopamine receptor as determined by radiation inactivation. Mol. Pharmacol. 1983;24:10–14. [PubMed] [Google Scholar]
- 83.Venter JC. Muscarinic cholinergic receptor structure. Receptor size, membrane orientation, and absence of major phylogenetic structural diversity. J. Biol. Chem. 1983;258:4842–4848. [PubMed] [Google Scholar]
- 84.Venter JC, Fraser CM. The structure of α- and β-adrenergic receptors. Trends Pharmacol. Sci. 1983;4:256–258. [Google Scholar]
- 85.Venter JC, Horne P, Eddy B, Greguski R, Fraser CM. α1-Adrenergic receptor structure. Mol. Pharmacol. 1984;26:196–205. [PubMed] [Google Scholar]
- 86.Venter JC, Schaber JS, U'Prichard DC, Fraser CM. Molecular size of the human platelet α2-adrenergic receptor as determined by radiation inactivation. Biochem. Biophys. Res. Commun. 1983;116:1070–1075. doi: 10.1016/s0006-291x(83)80251-4. [DOI] [PubMed] [Google Scholar]
- 87.Shirakawa O, Tanaka C. Molecular characterization of muscarinic receptor subtypes in bovine cerebral cortex by radiation inactivation and molecular exclusion HPLC. Br. J. Pharmacol. 1985;86:375–383. doi: 10.1111/j.1476-5381.1985.tb08906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bouvier C, Potier M, Beauregard G, Lafond J, Amlaiky N, Caron MG, Collu R. Solubilization and characterization of D2-dopamine receptors in an estrone-induced, prolactin-secreting rat pituitary adenoma. J. Neurochem. 1986;47:1653–1660. doi: 10.1111/j.1471-4159.1986.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 89.Kempner ES. Novel predictions from radiation target analysis. Trends Biochem. Sci. 1993;18:236–239. doi: 10.1016/0968-0004(93)90169-n. [DOI] [PubMed] [Google Scholar]
- 90.Salahpour A, Angers S, Bouvier M. Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol. Metab. 2000;11:163–168. doi: 10.1016/s1043-2760(00)00260-5. [DOI] [PubMed] [Google Scholar]
- 91.Milligan G. Oligomerisation of G-protein-coupled receptors. J. Cell Sci. 2001;114:1265–1271. doi: 10.1242/jcs.114.7.1265. [DOI] [PubMed] [Google Scholar]
- 92.Gomes I, Jordan BA, Gupta A, Rios C, Trapaidze N, Devi LA. G protein coupled receptor dimerization: Implications in modulating receptor function. J. Mol. Med. 2001;79:226–242. doi: 10.1007/s001090100219. [DOI] [PubMed] [Google Scholar]
- 93.George SR, O'Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discovery. 2002;1:808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 94.Gazi L, Lopez-Gimenez JF, Strange PG. Formation of oligomers by G protein-coupled receptors. Curr. Opin. Drug Discovery Dev. 2002;5:756–763. [PubMed] [Google Scholar]
- 95.Park P, Sum CS, Hampson DR, Van Tol HHM, Wells JW. Nature of the oligomers formed by muscarinic M2 acetylcholine receptors in Sf9 cells. Eur. J. Pharmacol. 2001;421:11–22. doi: 10.1016/s0014-2999(01)00998-0. [DOI] [PubMed] [Google Scholar]
- 96.Zeng F-Y, Wess J. Identification and molecular characterization of m3 muscarinic receptor dimers. J. Biol. Chem. 1999;274:19487–19497. doi: 10.1074/jbc.274.27.19487. [DOI] [PubMed] [Google Scholar]
- 97.Angers S, Salahpour A, Bouvier M. Dimerization: An emerging concept for G protein-coupled receptor ontogeny and function. Annu. Rev. Pharmacol. Toxicol. 2002;42:409–435. doi: 10.1146/annurev.pharmtox.42.091701.082314. [DOI] [PubMed] [Google Scholar]
- 98.Lee SP, O'Dowd BF, George SR. Homo- and hetero-oligomerization of G protein-coupled receptors. Life Sci. 2003;74:173–180. doi: 10.1016/j.lfs.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 99.Milligan G. G protein-coupled receptor dimerization: Function and ligand pharmacology. Mol. Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 100.Liang Y, Fotiadis D, Filipek S, Saperstein DA, Palczewski K, Engel A. Organization of the G protein-coupled receptors rhodopsin and opsin in native membranes. J. Biol. Chem. 2003;278:21655–21662. doi: 10.1074/jbc.M302536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chidiac P, Green MA, Pawagi AB, Wells JW. Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry. 1997;36:7361–7379. doi: 10.1021/bi961939t. [DOI] [PubMed] [Google Scholar]
- 102.Wreggett KA, Wells JW. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J. Biol. Chem. 1995;270:22488–22499. doi: 10.1074/jbc.270.38.22488. [DOI] [PubMed] [Google Scholar]
- 103.Park PS-H, Sum CS, Pawagi AB, Wells JW. Cooperativity and oligomeric status of cardiac muscarinic cholinergic receptors. Biochemistry. 2002;41:5588–5604. doi: 10.1021/bi011746s. [DOI] [PubMed] [Google Scholar]
- 104.Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 105.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. Quantitative assessment of β1- and β2-adrenergic receptor homo- and heterodimerization by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 106.Klco JM, Lassere TB, Baranski TJ. C5a receptor oligomerization. I. Disulfide trapping reveals oligomers and potential contact surfaces in a G protein-coupled receptor. J. Biol. Chem. 2003;278:35345–35353. doi: 10.1074/jbc.M305606200. [DOI] [PubMed] [Google Scholar]
- 107.Park PS-H, Wells JW. Oligomeric potential of the M2 muscarinic cholinergic receptor. J. Neurochem. 2004;90:537–548. doi: 10.1111/j.1471-4159.2004.02536.x. [DOI] [PubMed] [Google Scholar]
- 108.Terrillon S, Bouvier M. Roles of G-protein-coupled receptor dimerization. EMBO Rep. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. Homodimerization of the β2-adrenergic receptor as a prerequisite for cell surface targeting. J. Biol. Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 110.Lee SP, O'Dowd BF, Ng GY, Varghese G, Akil H, Mansour A, Nguyen T, George SR. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol. Pharmacol. 2000;58:120–128. doi: 10.1124/mol.58.1.120. [DOI] [PubMed] [Google Scholar]
- 111.Le Gouill C, Parent JL, Caron C-A, Gaudreau R, Volkov L, Rola-Pleszczynski M, Stankova J. Selective modulation of wild-type receptor functions by mutants of G-protein-coupled receptors. J. Biol. Chem. 1999;274:12548–12554. doi: 10.1074/jbc.274.18.12548. [DOI] [PubMed] [Google Scholar]
- 112.Zhu X, Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–15784. doi: 10.1021/bi981162z. [DOI] [PubMed] [Google Scholar]
- 113.Overton MC, Chinault SL, Blumer KJ. Oligomerization, biogenesis, and signaling is promoted by a glycophorin A-like dimerization motif in transmembrane domain 1 of a yeast G protein-coupled receptor. J. Biol. Chem. 2003;278:49369–49377. doi: 10.1074/jbc.M308654200. [DOI] [PubMed] [Google Scholar]
- 114.Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol. Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 115.Javitch JA. The ants go marching two by two: Oligomeric structure of G-protein-coupled receptors. Mol. Pharmacol. 2004 doi: 10.1124/mol.104.006320. in press. [DOI] [PubMed] [Google Scholar]
- 116.Carrillo JJ, Lopez-Gimenez JF, Milligan G. Multiple interactions between transmembrane helices generate the oligomeric α1b-adrenoceptor. Mol. Pharmacol. 2004 doi: 10.1124/mol.104.001586. in press. [DOI] [PubMed] [Google Scholar]
- 117.Guo W, Shi L, Javitch JA. The fourth transmembrane segment forms the interface of the dopamine D2 receptor homodimer. J. Biol. Chem. 2003;278:4385–4388. doi: 10.1074/jbc.C200679200. [DOI] [PubMed] [Google Scholar]
- 118.Lee SP, O'Dowd BF, Rajaram RD, Nguyen T, George SR. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry. 2003;42:11023–11031. doi: 10.1021/bi0345539. [DOI] [PubMed] [Google Scholar]
- 119.Dean MK, Higgs C, Smith RE, Bywater RP, Snell CR, Scott PD, Upton GJ, Howe TJ, Reynolds CA. Dimerization of G-protein-coupled receptors. J. Med. Chem. 2001;44:4595–4614. doi: 10.1021/jm010290+. [DOI] [PubMed] [Google Scholar]
- 120.Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett. 2004;564:281–288. doi: 10.1016/S0014-5793(04)00194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kroeger KM, Pfleger KD, Eidne KA. G-protein coupled receptor oligomerization in neuroendocrine pathways. Front. Neuroendocrinol. 2003;24:254–278. doi: 10.1016/j.yfrne.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 122.Maggio R, Vogel Z, Wess J. Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular “cross-talk” between G-protein-linked receptors. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3103–3107. doi: 10.1073/pnas.90.7.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maggio R, Barbier P, Fornai F, Corsini GU. Functional role of the third cytoplasmic loop in muscarinic receptor dimerization. J. Biol. Chem. 1996;271:31055–31060. doi: 10.1074/jbc.271.49.31055. [DOI] [PubMed] [Google Scholar]
- 124.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 125.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 126.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: Formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 127.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of m and d opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galvez T, Duthey B, Kniazeff J, Blahos J, Rovelli G, Bettler B, Prezeau L, Pin JP. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABAB receptor function. EMBO J. 2001;20:2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 130.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 131.Calver AR, Robbins MJ, Cosio C, Rice SQ, Babbs AJ, Hirst WD, Boyfield I, Wood MD, Russell RB, Price GW, Couve A, Moss SJ, Pangalos MN. The C-terminal domains of the GABAB receptor subunits mediate intracellular trafficking but are not required for receptor signaling. J. Neurosci. 2001;21:1203–1210. doi: 10.1523/JNEUROSCI.21-04-01203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pagano A, Rovelli G, Mosbacher J, Lohmann T, Duthey B, Stauffer D, Ristig D, Schuler V, Meigel I, Lampert C, Stein T, Prezeau L, Blahos J, Pin J, Froestl W, Kuhn R, Heid J, Kaupmann K, Bettler B. C-Terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABAB receptors. J. Neurosci. 2001;21:1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim. Biophys. Acta. 1990;1031:163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- 134.Gilman AG. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 135.Stryer L. Cyclic GMP cascade of vision. Annu. Rev. Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 136.Lefkowitz RJ. Historical review: A brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 2004;25:413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 137.Kenakin T. Principles: Receptor theory in pharmacology. Trends Pharmacol. Sci. 2004;25:186–192. doi: 10.1016/j.tips.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 138.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled β-adrenergic receptor. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 139.Wreggett KA, De Lean A. The ternary complex model. Its properties and application to ligand interactions with the D2-dopamine receptor of the anterior pituitary gland. Mol. Pharmacol. 1984;26:214–227. [PubMed] [Google Scholar]
- 140.Leung E, Jacobson KA, Green RD. Analysis of agonist-antagonist interactions at A1 adenosine receptors. Mol. Pharmacol. 1990;38:72–83. [PMC free article] [PubMed] [Google Scholar]
- 141.Sibley DR, De Lean A, Creese I. Anterior pituitary dopamine receptors. Demonstration of interconvertible high and low affinity states of the D2 dopamine receptor. J. Biol. Chem. 1982;257:6351–6361. [PubMed] [Google Scholar]
- 142.Ehlert FJ. The relationship between muscarinic receptor occupancy and adenylate cyclase inhibition in the rabbit myocardium. Mol. Pharmacol. 1985;28:410–421. [PubMed] [Google Scholar]
- 143.Kent RS, De Lean A, Lefkowitz RJ. A quantitative analysis of β-adrenergic receptor interactions: Resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol. Pharmacol. 1980;17:14–23. [PubMed] [Google Scholar]
- 144.Birdsall NJM, Burgen ASV, Hulme EC. Correlation between the binding properties and pharmacological responses of muscarinic receptors. Adv. Behav. Biol. 1977;24:25–33. [Google Scholar]
- 145.Potter LT, Ferrendelli CA. Affinities of different cholinergic agonists for the high and low affinity states of hippocampal M1 muscarine receptors. J. Pharmacol. Exp. Ther. 1989;248:974–978. [PubMed] [Google Scholar]
- 146.Evans T, Hepler JR, Masters SB, Brown JH, Harden TK. Guanine nucleotide regulation of agonist binding to muscarinic cholinergic receptors. Relation to efficacy of agonists for stimulation of phosphoinositide breakdown and Ca2+ mobilization. Biochem. J. 1985;232:751–757. doi: 10.1042/bj2320751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chidiac P, Nagy A, Sole MJ, Wells JW. Inefficient muscarinic transduction in cardiomyopathic Syrian hamsters. J. Mol. Cell. Cardiol. 1991;23:1255–1269. doi: 10.1016/0022-2828(91)90083-x. [DOI] [PubMed] [Google Scholar]
- 148.Flynn DD, Weinstein DA, Mash DC. Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer's disease: Implications for the failure of cholinergic replacement therapies. Ann. Neurol. 1991;29:256–262. doi: 10.1002/ana.410290305. [DOI] [PubMed] [Google Scholar]
- 149.Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 150.Green MA, Chidiac P, Wells JW. Cardiac muscarinic receptors: Relationship between the G protein and multiple states of affinity. Biochemistry. 1997;36:7380–7394. doi: 10.1021/bi961940s. [DOI] [PubMed] [Google Scholar]
- 151.Lee TWT, Sole MJ, Wells JW. Assessment of a ternary model for the binding of agonists to neurohumoral receptors. Biochemistry. 1986;25:7009–7020. doi: 10.1021/bi00370a038. [DOI] [PubMed] [Google Scholar]
- 152.Sinkins WG, Wells JW. G protein-linked receptors labeled by [3H]histamine in guinea pig cerbral cortex. II. Mechanistic basis for multiple states of affinity. Mol. Pharmacol. 1993;43:583–594. [PubMed] [Google Scholar]
- 153.Chidiac P. Rethinking receptor-G protein-effector interactions. Biochem. Pharmacol. 1998;55:549–556. doi: 10.1016/s0006-2952(97)00361-4. [DOI] [PubMed] [Google Scholar]
- 154.Munson PJ, Rodbard D. Ligand: A versatile computerized approach for characterization of ligand-binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 155.Wong H-MS, Sole MJ, Wells JW. Assessment of mechanistic proposals for the binding of agonists to cardiac muscarinic receptors. Biochemistry. 1986;25:6995–7008. doi: 10.1021/bi00370a037. [DOI] [PubMed] [Google Scholar]
- 156.Neubig RR. Membrane organization in G-protein mechanisms. FASEB J. 1994;8:939–946. doi: 10.1096/fasebj.8.12.8088459. [DOI] [PubMed] [Google Scholar]
- 157.Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors. Annu. Rev. Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- 158.Lu ZL, Saldanha JW, Hulme EC. Seven-transmembrane receptors: Crystals clarify. Trends Pharmacol. Sci. 2002;23:140–146. doi: 10.1016/S0165-6147(00)01973-8. [DOI] [PubMed] [Google Scholar]
- 159.Peterson GL, Herron GS, Yamaki M, Fullerton DS, Schimerlik MI. Purification of the muscarinic acetylcholine receptor from porcine atria. Proc. Natl. Acad. Sci. U.S.A. 1984;81:4993–4997. doi: 10.1073/pnas.81.15.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Boyer JL, Martinez-Carcamo M, Monroy-Sanchez JA, Posadas C, Garcia-Sainz JA. Guanine nucleotide-induced positive cooperativity in muscarinic-cholinergic antagonist binding. Biochem. Biophys. Res. Commun. 1986;134:172–177. doi: 10.1016/0006-291x(86)90543-7. [DOI] [PubMed] [Google Scholar]
- 161.Henis YI, Sokolovsky M. Muscarinic antagonists induce different receptor conformations in rat adenohypophysis. Mol. Pharmacol. 1983;24:357–365. [PubMed] [Google Scholar]
- 162.Limbird LE, De Meyts P, Lefkowitz RJ. β-Adrenergic receptors: Evidence for negative cooperativity. Biochem. Biophys. Res. Commun. 1975;64:1160–1168. doi: 10.1016/0006-291x(75)90815-3. [DOI] [PubMed] [Google Scholar]
- 163.Mattera R, Pitts BJ, Entman ML, Birnbaumer L. Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. Evidence for homo- and heterotropic cooperativity in ligand binding analyzed by computer-assisted curve fitting. J. Biol. Chem. 1985;260:7410–7421. [PubMed] [Google Scholar]
- 164.Sinkins WG, Kandel M, Kandel SI, Schunack W, Wells JW. G protein-linked receptors labeled by [3H]histamine in guinea pig cerebral cortex. I. Pharmacological characterization. Mol. Pharmacol. 1993;43:569–582. [PubMed] [Google Scholar]
- 165.Steinberg GH, Eppel JG, Kandel M, Kandel SI, Wells JW. H2 histaminic receptors in rat cerebral cortex. 1. Binding of [3H]histamine. Biochemistry. 1985;24:6095–6107. doi: 10.1021/bi00343a011. [DOI] [PubMed] [Google Scholar]
- 166.Steinberg GH, Kandel M, Kandel SI, Wells JW. H2 histaminic receptors in rat cerebral cortex. 2. Inhibition of [3H]histamine by H2 antagonists. Biochemistry. 1985;24:6107–6115. doi: 10.1021/bi00343a012. [DOI] [PubMed] [Google Scholar]
- 167.Steinberg GH, Kandel M, Kandel SI, Wells JW. H2 histaminic receptors in rat cerebral cortex. 3. Inhibition of [3H]histamine by H2 agonists. Biochemistry. 1985;24:6115–6125. doi: 10.1021/bi00343a013. [DOI] [PubMed] [Google Scholar]
- 168.Christopoulos A, Kenakin T. G Protein-Coupled Receptor Allosterism and Complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 169.Armstrong D, Strange PG. Dopamine D2 receptor dimer formation: Evidence from ligand binding. J. Biol. Chem. 2001;276:22621–22629. doi: 10.1074/jbc.M006936200. [DOI] [PubMed] [Google Scholar]
- 170.Jordan BA, Devi LA. G-Protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lavoie C, Hebert TE. Pharmacological characterization of putative β1-β2-adrenergic receptor heterodimers. Can. J. Physiol. Pharmacol. 2003;81:186–195. doi: 10.1139/y02-167. [DOI] [PubMed] [Google Scholar]
- 172.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 173.Koshland DE, Jr., Nemethy G, Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 174.Kjelsberg MA, Cotecchia S, Ostrowski J, Caron MG, Lefkowitz RJ. Constitutive activation of the α1B-adrenergic receptor by all amino acid substitutions at a single site. Evidence for a region which constrains receptor activation. J. Biol. Chem. 1992;267:1430–1433. [PubMed] [Google Scholar]
- 175.Spalding TA, Burstein ES, Wells JW, Brann MR. Constitutive activation of the M5 muscarinic receptor by a series of mutations at the extracellular end of transmembrane 6. Biochemistry. 1997;36:10109–10116. doi: 10.1021/bi970565g. [DOI] [PubMed] [Google Scholar]
- 176.Samama P, Pei G, Costa T, Cotecchia S, Lefkowitz RJ. Negative antagonists promote an inactive conformation of the β2-adrenergic receptor. Mol. Pharmacol. 1994;45:390–394. [PubMed] [Google Scholar]
- 177.Strange PG. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol. Sci. 2002;23:89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- 178.Kenakin T. Inverse, protean, and ligand-selective agonism: Matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- 179.Milligan G. Constitutive activity and inverse agonists of G protein-coupled receptors: A current perspective. Mol. Pharmacol. 2003;64:1271–1276. doi: 10.1124/mol.64.6.1271. [DOI] [PubMed] [Google Scholar]
- 180.Sum CS, Park PS-H, Wells JW. Effects of N-ethylmaleimide on conformational equilibria in purified cardiac muscarinic receptors. J. Biol. Chem. 2002;277:36188–36203. doi: 10.1074/jbc.M201731200. [DOI] [PubMed] [Google Scholar]
- 181.Ridge KD, Abdulaev NG, Sousa M, Palczewski K. Phototransduction: Crystal clear. Trends Biochem. Sci. 2003;28:479–487. doi: 10.1016/S0968-0004(03)00172-5. [DOI] [PubMed] [Google Scholar]
- 182.Pierce KL, Lefkowitz RJ. Classical and new roles of β-arrestins in the regulation of G-protein-coupled receptors. Nat. Rev. Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 183.Baneres JL, Parello J. Structure-based analysis of GPCR function: Evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. J. Mol. Biol. 2003;329:815–829. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- 184.Wessling-Resnick M, Johnson GL. Transducin interactions with rhodopsin. Evidence for positive cooperative behavior. J. Biol. Chem. 1987;262:12444–12447. [PubMed] [Google Scholar]
- 185.Willardson BM, Pou B, Yoshida T, Bitensky MW. Cooperative binding of the retinal rod G-protein, transducin, to light-activated rhodopsin. J. Biol. Chem. 1993;268:6371–6382. [PubMed] [Google Scholar]
- 186.Bitensky MW, Wheeler GL, Aloni B, Vetury S, Matuo Y. Light- and GTP-activated photoreceptor phosphodiesterase: Regulation by a light-activated GTPase and identification of rhodopsin as the phosphodiesterase binding site. Adv. Cyclic Nucleotide Res. 1978;9:553–572. [PubMed] [Google Scholar]
- 187.Chidiac P, Wells JW. Effects of adenyl nucleotides and carbachol on cooperative interactions among G proteins. Biochemistry. 1992;31:10908–10921. doi: 10.1021/bi00159a035. [DOI] [PubMed] [Google Scholar]
- 188.Tota MR, Kahler KR, Schimerlik MI. Reconstitution of the purified porcine atrial muscarinic acetylcholine receptor with purified porcine atrial inhibitory guanine nucleotide binding protein. Biochemistry. 1987;26:8175–8182. doi: 10.1021/bi00399a023. [DOI] [PubMed] [Google Scholar]
- 189.Hilf G, Gierschik P, Jakobs KH. Muscarinic acetylcholine receptor-stimulated binding of guanosine 5′-O-(3-thiotriphosphate) to guanine-nucleotide-binding proteins in cardiac membranes. Eur. J. Biochem. 1989;186:725–731. doi: 10.1111/j.1432-1033.1989.tb15266.x. [DOI] [PubMed] [Google Scholar]
- 190.Baehr W, Morita EA, Swanson RJ, Applebury ML. Characterization of bovine rod outer segment G-protein. J. Biol. Chem. 1982;257:6452–6460. [PubMed] [Google Scholar]
- 191.Schlegel W, Kempner ES, Rodbell M. Activation of adenylate cyclase in hepatic membranes involves interactions of the catalytic unit with multimeric complexes of regulatory proteins. J. Biol. Chem. 1979;254:5168–5176. [PubMed] [Google Scholar]
- 192.Coulter S, Rodbell M. Heterotrimeric G proteins in synaptoneurosome membranes are cross-linked by p-phenylenedimaleimide, yielding structures comparable in size to cross-linked tubulin and F-actin. Proc. Natl. Acad. Sci. U.S.A. 1992;89:5842–5846. doi: 10.1073/pnas.89.13.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hingorani VN, Tobias DT, Henderson JT, Ho YK. Chemical cross-linking of bovine retinal transducin and cGMP phosphodiesterase. J. Biol. Chem. 1988;263:6916–6926. [PubMed] [Google Scholar]
- 194.Mixon MB, Lee E, Coleman DE, Berghuis AM, Gilman AG, Sprang SR. Tertiary and quaternary structural changes in Gia1 induced by GTP hydrolysis. Science. 1995;270:954–960. doi: 10.1126/science.270.5238.954. [DOI] [PubMed] [Google Scholar]
- 195.Wessling-Resnick M, Johnson GL. Evidence for oligomeric forms of transducin's α subunit: Formation of intermolecular α–α disulfide linkages. Biochem. Biophys. Res. Commun. 1989;159:651–657. doi: 10.1016/0006-291x(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 196.Vaillancourt RR, Dhanasekaran N, Johnson GL, Ruoho AE. 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: Evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus of α interacts with both α and γ subunits. Proc. Natl. Acad. Sci. U.S.A. 1990;87:3645–3649. doi: 10.1073/pnas.87.10.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jahangeer S, Rodbell M. The disaggregation theory of signal transduction revisited: Further evidence that G proteins are multimeric and disaggregate to monomers when activated. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8782–8786. doi: 10.1073/pnas.90.19.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kobilka BK. Agonist-induced conformational changes in the β2 adrenergic receptor. J. Pept. Res. 2002;60:317–321. doi: 10.1034/j.1399-3011.2002.21062.x. [DOI] [PubMed] [Google Scholar]
- 199.Karnik SS, Gogonea C, Patil S, Saad Y, Takezako T. Activation of G-protein-coupled receptors: A common molecular mechanism. Trends Endocrinol. Metab. 2003;14:431–437. doi: 10.1016/j.tem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 200.Parnot C, Kobilka B. Toward understanding GPCR dimers. Nat. Struct. Mol. Biol. 2004;11:691–692. doi: 10.1038/nsmb0804-691. [DOI] [PubMed] [Google Scholar]
- 201.Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. Ligand-induced rearrangement of the dimeric metabotropic glutamate receptor 1a. Nat. Struct. Mol. Biol. 2004;11:637–642. doi: 10.1038/nsmb770. [DOI] [PubMed] [Google Scholar]
- 202.Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prezeau L, Pin JP. Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat. Struct. Mol. Biol. 2004;11:706–713. doi: 10.1038/nsmb794. [DOI] [PubMed] [Google Scholar]
- 203.Perutz MF. Mechanisms of cooperativity and allosteric regulation in proteins. Q. Rev. Biophys. 1989;22:139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 204.Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fotiadis D, Scheuring S, Müller SA, Engel A, Müller DJ. Imaging and manipulation of biological structures with the AFM. Micron. 2002;33:385–397. doi: 10.1016/s0968-4328(01)00026-9. [DOI] [PubMed] [Google Scholar]
- 206.Müller DJ, Sass H-J, Müller SA, Buldt G, Engel A. Surface structures of native bacteriorhodopsin depend on the molecular packing arrangement in the membrane. J. Mol. Biol. 1999;285:1903–1909. doi: 10.1006/jmbi.1998.2441. [DOI] [PubMed] [Google Scholar]
- 207.Oesterhelt F, Oesterhelt D, Pfeiffer M, Engel A, Gaub HE, Müller DJ. Unfolding pathways of individual bacteriorhodopsins. Science. 2000;288:143–146. doi: 10.1126/science.288.5463.143. [DOI] [PubMed] [Google Scholar]
- 208.Baumeister W. Electron tomography: Towards visualizing the molecular organization of the cytoplasm. Curr. Opin. Struct. Biol. 2002;12:679–684. doi: 10.1016/s0959-440x(02)00378-0. [DOI] [PubMed] [Google Scholar]
- 209.Wyman J. Allosteric linkage. J. Am. Chem. Soc. 1967;89:2202–2218. [Google Scholar]
- 210.Neubig RR, Gantzos RD, Brasier RS. Agonist and antagonist binding to α2-adrenergic receptors in purified membranes from human platelets. Implications of receptor-inhibitory nucleotide-binding protein stoichiometry. Mol. Pharmacol. 1985;28:475–486. [PubMed] [Google Scholar]
- 211.Jastrzebska B, Maeda T, Zhu L, Fotiadis D, Filipek S, Engel A, Stenkamp RE, Palczewski K. Functional characterization of rhodopsin monomers and dimers in detergents. J. Biol. Chem. 2004 doi: 10.1074/jbc.M408691200. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]



