Abstract
OBJECTIVE—To characterise the type and kinetics of T cell clones in synovial lesions of patients with rheumatoid arthritis (RA). METHODS—Mononuclear cells from serial samples of synovial fluid (SF) and peripheral blood from nine RA patients were separated phenotypically using antibody coated magnetic beads. After mRNA preparation, reverse transcription-polymerase chain reaction (RT-PCR) was performed to amplify V-D(N)-J (that is, the third complementarity determining, CDR3) regions of their T cell receptor beta chain genes. This was followed by single strand conformation polymorphism (SSCP) analysis to detect the clonotypes of accumulating T cells. Amino acid sequences of the dominant clones were also determined. RESULTS—Although peripheral T cells were heterogeneous, accumulation of oligoclonal T cells was detected in SF. The predominant accumulating clone was the CD8 subset, which was persistently present in serial samples obtained over almost one year of follow up. A proportion of these cells expressed CD25 or CD45RO, or both, suggesting they are `memory' clones. CONCLUSION—The persistent presence of CD8+ T cell clones in RA joints indicates that they may be involved in the perpetuation of the chronic inflammatory process in RA joints.
Full Text
The Full Text of this article is available as a PDF (113.7 KB).
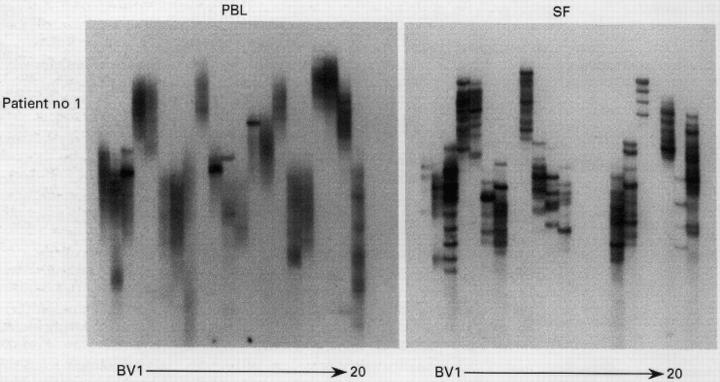
Figure 1 .
RT-PCR-SSCP analysis of T cells clonality of peripheral blood lymphocytes (PBL) and synovial fluid (SF) in a representative patient with rheumatoid arthritis. Results of final SSCP are displayed. Lanes: from left to right, BV 1 to 20 including BVSSI/BVSS2 and BV13SI/BV13S2 subfamilies.
Figure 2 .
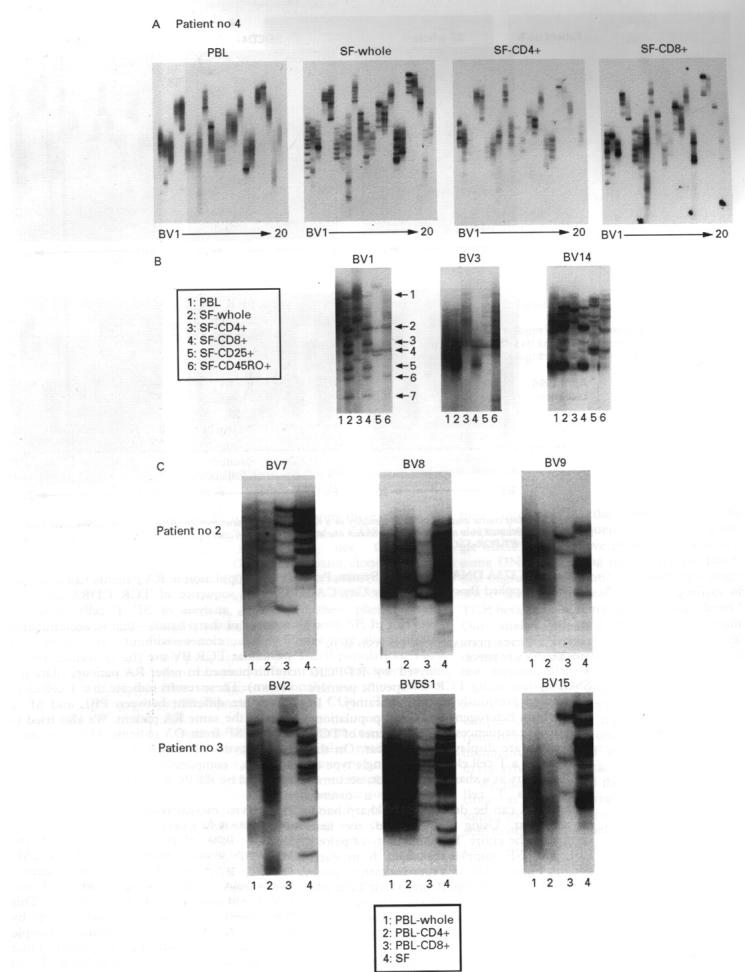
Dominant clonal accumulations are demonstrated in CD8+ T cell subset of synovial T cells. (A) Results of analysis of T cell clonality of peripheral blood lymphocytes (PBL) and synovial fluid T cells (SFTC) of a representative patient with rheumatoid arthritis. SFTC were examined as a whole group, CD4+ and CD8+ populations, and analysed independently. (B) Amplified DNAs from each population were electrophoresised on the same SSCP gel to provide a comparison between the groups. (C) Results of T cell clonality analyses of PBL and SFTC, with a phenotypic separation of PBL.
Figure 3 .
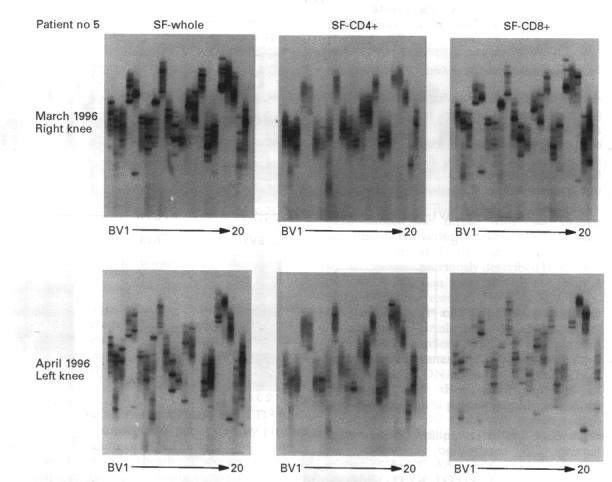
A time course analysis of T cell clonality in a representative patient with rheumatoid arthritis. Synovial fluid samples were obtained from the same patient within one month from the left and right knee joints and analysed for T cell clonality using RT-PCR-SSCP.
Figure 4 .
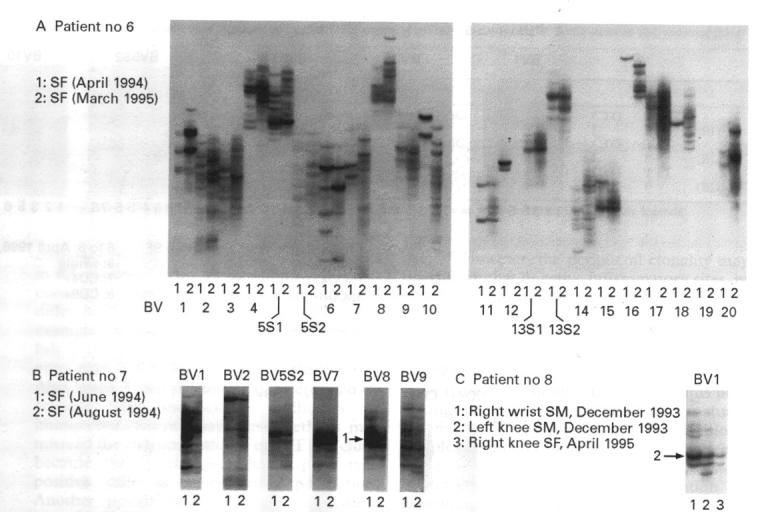
Comparison of accumulating T cell clones in synovial fluid (SF) in two patients with rheumatoid arthritis at different time intervals. (A) and (B) SF T cell samples obtained at the indicated time intervals from two patients were individually analysed by RT-PCR-SSCP and finally electrophoresised on the same SSCP gel. (C) Two specimens of synovial membranes from the right wrist and left knee joint were obtained from another patient in 1993, followed by samples of synovial fluid from the right knee joint in 1995. Each sample was analysed independently and compared on the same SSCP gel. Arrows indicate dominantly accumulating clones.
Figure 5 .
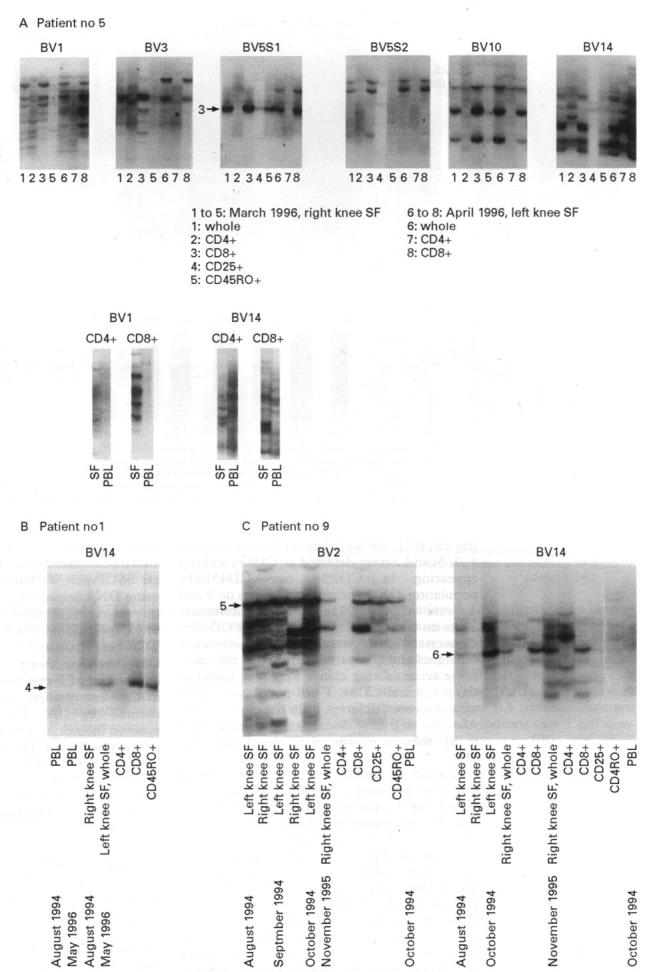
Serial determination of accumulated T cell clones and their phenotype. (A) to (C) Serial samples of synovial fluid T cells and peripheral blood lymphocyte (PBL) were obtained from three RA patients and separated into T cell subsets. The detected clones were compared with each other by electrophoresising to the same SSCP gels. Arrows indicate dominantly accumulating clones. The amino acid sequences of the bands are shown in table 3.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam A., Lambert N., Lulé J., Coppin H., Mazières B., de Préval C., Cantagrel A. Persistence of dominant T cell clones in synovial tissues during rheumatoid arthritis. J Immunol. 1996 May 1;156(9):3480–3485. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Roessner K. D., Naito-Hoopes M., Howard D. B., Gaur L. K., Budd R. C. Increased usage of V beta 2 and V beta 6 in rheumatoid synovial fluid T cells. Arthritis Rheum. 1994 Nov;37(11):1627–1636. doi: 10.1002/art.1780371112. [DOI] [PubMed] [Google Scholar]
- David-Ameline J., Lim A., Davodeau F., Peyrat M. A., Berthelot J. M., Semana G., Pannetier C., Gaschet J., Vie H., Even J. Selection of T cells reactive against autologous B lymphoblastoid cells during chronic rheumatoid arthritis. J Immunol. 1996 Nov 15;157(10):4697–4706. [PubMed] [Google Scholar]
- DerSimonian H., Sugita M., Glass D. N., Maier A. L., Weinblatt M. E., Rème T., Brenner M. B. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993 Jun 1;177(6):1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. E., Ricalton N. S., Meyer A. C., West S. G., Kaplan H., Behrendt C., Kotzin B. L. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995 Apr 1;154(7):3538–3547. [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Goronzy J. J., Bartz-Bazzanella P., Hu W., Jendro M. C., Walser-Kuntz D. R., Weyand C. M. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest. 1994 Nov;94(5):2068–2076. doi: 10.1172/JCI117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Uchida S. T cytotoxic and helper cells are markedly increased, and T suppressor and inducer cells are markedly decreased, in rheumatoid synovial fluids. Arthritis Rheum. 1987 Jul;30(7):737–743. doi: 10.1002/art.1780300703. [DOI] [PubMed] [Google Scholar]
- Grom A. A., Thompson S. D., Luyrink L., Passo M., Choi E., Glass D. N. Dominant T-cell-receptor beta chain variable region V beta 14+ clones in juvenile rheumatoid arthritis. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11104–11108. doi: 10.1073/pnas.90.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson S., Rönnelid J., Karlsson-Parra A., Lysholm J., Gudbjörnsson B., Widenfalk B., Janson C. H., Klareskog L. T-cell receptor V-gene usage in synovial fluid and synovial tissue from RA patients. Scand J Immunol. 1992 Nov;36(5):681–688. doi: 10.1111/j.1365-3083.1992.tb03128.x. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Choi I. H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P. K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993 Nov 15;151(10):5762–5769. [PubMed] [Google Scholar]
- Hingorani R., Monteiro J., Furie R., Chartash E., Navarrete C., Pergolizzi R., Gregersen P. K. Oligoclonality of V beta 3 TCR chains in the CD8+ T cell population of rheumatoid arthritis patients. J Immunol. 1996 Jan 15;156(2):852–858. [PubMed] [Google Scholar]
- Höger T. A., Tokuyama M., Yonamine K., Hayashi K., Masuko-Hongo K., Kato T., Kobata T., Mizushima Y., Nishioka K., Yamamoto K. Time course analysis of alpha+ beta+ T cell clones during normal pregnancy. Eur J Immunol. 1996 Apr;26(4):834–838. doi: 10.1002/eji.1830260416. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Masuko K., Nakai Y., Kato T., Hasanuma T., Yoshino S. I., Mizushima Y., Nishioka K., Yamamoto K. High frequencies of identical T cell clonotypes in synovial tissues of rheumatoid arthritis patients suggest the occurrence of common antigen-driven immune responses. Arthritis Rheum. 1996 Mar;39(3):446–453. doi: 10.1002/art.1780390312. [DOI] [PubMed] [Google Scholar]
- Khazaei H. A., Lunardi C., So A. K. CD4 T cells in the rheumatoid joint are oligoclonally activated and change during the course of disease. Ann Rheum Dis. 1995 Apr;54(4):314–317. doi: 10.1136/ard.54.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryliszyn-Moskal A. Comparison of blood and synovial fluid lymphocyte subsets in rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 1995 Jan;14(1):43–50. doi: 10.1007/BF02208083. [DOI] [PubMed] [Google Scholar]
- Li Y., Sun G. R., Tumang J. R., Crow M. K., Friedman S. M. CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest. 1994 Dec;94(6):2525–2531. doi: 10.1172/JCI117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko K., Kato S., Hagihara M., Tsuchida F., Takemoto Y., Izawa K., Kato T., Yamamori S., Mizushima Y., Nishioka K. Stable clonal expansion of T cells induced by bone marrow transplantation. Blood. 1996 Jan 15;87(2):789–799. [PubMed] [Google Scholar]
- Masuko K., Kato T., Ikeda Y., Okubo M., Mizushima Y., Nishioka K., Yamamoto K. Dynamic changes of accumulated T cell clonotypes during antigenic stimulation in vivo and in vitro. Int Immunol. 1994 Dec;6(12):1959–1966. doi: 10.1093/intimm/6.12.1959. [DOI] [PubMed] [Google Scholar]
- Matthews N., Emery P., Pilling D., Akbar A., Salmon M. Subpopulations of primed T helper cells in rheumatoid arthritis. Arthritis Rheum. 1993 May;36(5):603–607. doi: 10.1002/art.1780360505. [DOI] [PubMed] [Google Scholar]
- Nakao H., Eguchi K., Kawakami A., Migita K., Otsubo T., Ueki Y., Shimomura C., Tezuka H., Matsunaga M., Maeda K. Phenotypic characterization of lymphocytes infiltrating synovial tissue from patients with rheumatoid arthritis: analysis of lymphocytes isolated from minced synovial tissue by dual immunofluorescent staining. J Rheumatol. 1990 Feb;17(2):142–148. [PubMed] [Google Scholar]
- Okumura M., Fujii Y., Inada K., Nakahara K., Matsuda H. Both CD45RA+ and CD45RA- subpopulations of CD8+ T cells contain cells with high levels of lymphocyte function-associated antigen-1 expression, a phenotype of primed T cells. J Immunol. 1993 Jan 15;150(2):429–437. [PubMed] [Google Scholar]
- Rabin R. L., Roederer M., Maldonado Y., Petru A., Herzenberg L. A., Herzenberg L. A. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest. 1995 May;95(5):2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S. J., Jones R. A., Roberts B. E., Patel D., Scott C. S. Relationships between 2H4 (CD45RA) and UCHL1 (CD45RO) expression by normal blood CD4+CD8-, CD4-CD8+, CD4-CD8dim+, CD3+CD4-CD8- and CD3-CD4-CD8- lymphocytes. Clin Exp Immunol. 1990 Jul;81(1):149–155. doi: 10.1111/j.1365-2249.1990.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. C. T cell receptor usage in rheumatic disease. Clin Exp Rheumatol. 1992 May-Jun;10(3):271–283. [PubMed] [Google Scholar]
- Roederer M., Dubs J. G., Anderson M. T., Raju P. A., Herzenberg L. A., Herzenberg L. A. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995 May;95(5):2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottini A., Imberti L., Bettinardi A., Mazza C., Gorla R., Primi D. Selection of T lymphocytes in two rheumatoid arthritis patients defines different T-cell receptor V beta repertoires in CD4+ and CD8+ T-cell subsets. J Autoimmun. 1993 Oct;6(5):621–637. doi: 10.1006/jaut.1993.1051. [DOI] [PubMed] [Google Scholar]
- Struyk L., Hawes G. E., Chatila M. K., Breedveld F. C., Kurnick J. T., van den Elsen P. J. T cell receptors in rheumatoid arthritis. Arthritis Rheum. 1995 May;38(5):577–589. doi: 10.1002/art.1780380502. [DOI] [PubMed] [Google Scholar]
- Struyk L., Hawes G. E., Dolhain R. J., van Scherpenzeel A., Godthelp B., Breedveld F. C., van den Elsen P. J. Evidence for selective in vivo expansion of synovial tissue-infiltrating CD4+ CD45RO+ T lymphocytes on the basis of CDR3 diversity. Int Immunol. 1994 Jun;6(6):897–907. doi: 10.1093/intimm/6.6.897. [DOI] [PubMed] [Google Scholar]
- Waase I., Kayser C., Carlson P. J., Goronzy J. J., Weyand C. M. Oligoclonal T cell proliferation in patients with rheumatoid arthritis and their unaffected siblings. Arthritis Rheum. 1996 Jun;39(6):904–913. doi: 10.1002/art.1780390606. [DOI] [PubMed] [Google Scholar]
- Wang E. C., Lawson T. M., Vedhara K., Moss P. A., Lehner P. J., Borysiewicz L. K. CD8high+ (CD57+) T cells in patients with rheumatoid arthritis. Arthritis Rheum. 1997 Feb;40(2):237–248. doi: 10.1002/art.1780400208. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Sakoda H., Nakajima T., Kato T., Okubo M., Dohi M., Mizushima Y., Ito K., Nishioka K. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol. 1992 Nov;4(11):1219–1223. doi: 10.1093/intimm/4.11.1219. [DOI] [PubMed] [Google Scholar]
- Zola H., Flego L., Macardle P. J., Donohoe P. J., Ranford J., Roberton D. The CD45RO (p180, UCHL1) marker: complexity of expression in peripheral blood. Cell Immunol. 1992 Nov;145(1):175–186. doi: 10.1016/0008-8749(92)90321-f. [DOI] [PubMed] [Google Scholar]