Abstract
OBJECTIVES—To test the hypothesis that patients with chronic inflammatory pain develop adaptive cortical responses to noxious stimulation characterised by reduced anterior cingulate responses. METHODS—Positron emission tomography was used to measure changes in regional cerebral blood flow (rCBF) in response to an acute experimental pain stimulus in six patients with rheumatoid arthritis (RA) in comparison to six age and sex matched controls. A standardised and reproducible non-painful and painful phasic heat stimulus was delivered by a thermal probe to the back of the right hand during six two minute periods during which time rCBF measurements were made. The effects of non-painful heat were subtracted from those of painful heat to weight the analysis towards the non-discriminatory or `suffering' components of pain processing. Significance maps of pain processing were generated and compared in each group and contrasted with results obtained in a group of patients with atypical facial pain (AFP) that have been previously published. RESULTS—The RA patients showed remarkably damped cortical and subcortical responses to pain compared with the control group. Significant differences between the two groups were observed in the prefrontal (BA 10) and anterior cingulate (BA 24 ) and cingulofrontal transition cortical (BA 32) areas. The reduced anterior cingulate responses to standardised heat pain were compared with the increased cingulate responses seen in patients with psychogenically maintained pain (AFP) who had both lower pain tolerance and mood than the RA group. CONCLUSIONS—Major cortical adaptive responses to standardised noxious heat can be measured and contrasted in patients with different types of chronic pain. The different pattern of cingulate and frontal cortical responses in the patients with inflammatory and non-nociceptive pain suggest that different mechanisms are operating, possibly at a thalamocortical level. Implications for treatment strategies for chronic pain are discussed.
Full Text
The Full Text of this article is available as a PDF (147.0 KB).
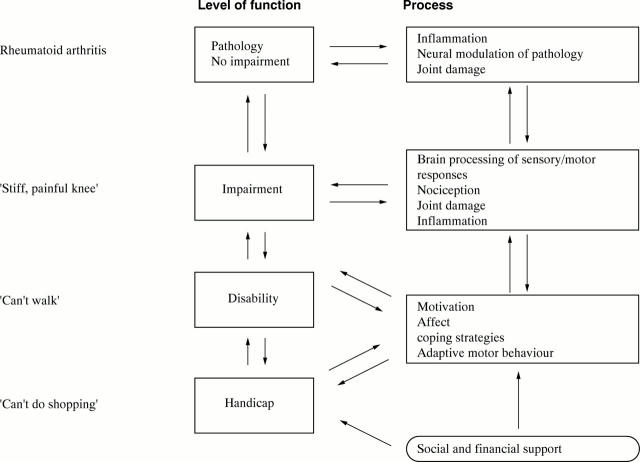
Figure 1 .
Schema of components of processes leading to handicap and the different components of disease related behaviour that may contribute to it.
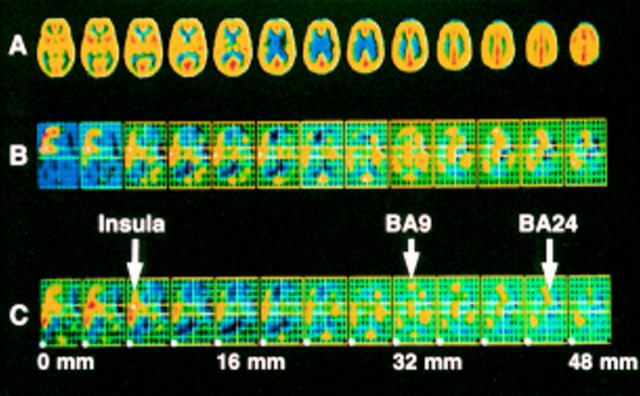
Figure 2 .
Subsignificant responses to the suffering components of experimental heat pain in a group of six patients with RA pain. After correction for global blood flow changes the data for the group were pooled and fitted to a standardised stereotactic template. Significant increases in rCBF as a result of the non-discriminatory (suffering) components of the pain response were assessed by subtracting the effect of non-painful heat stimulation from painful heat stimulation. At the top are transverse images of the brain after stereotaxic normalisation, with the distances from the AC-PC (anterior commissure-posterior commissure) plane indicated. (A) Anatomical features obtained by averaging all blood flow scans from the six female patients. (B) Arithmetical difference between adjusted mean blood flows for painful hot and non-painful hot stimuli. (C) The SPM (t) values derived from the formal pixel by pixel comparison of the adjusted mean blood flows and variances for each of the two conditions. The colour scale is arbitrary from low values to high, represented from mauve/blue, green, yellow, red to white respectively. Threshold significance is indicated by the lower left pixel for each plane and therefore only white is significant at a p<0.001 (Z threshold 3.09). Orientation: top is the front of the brain (rostral) and bottom is the back (caudal). Left is left. Arrows identify some of the subsignificant responses in left insula cortex, medial prefrontal cortex, (BA9) and anterior cingulate cortex (BA 24).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affleck G., Urrows S., Tennen H., Higgins P., Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996 Dec;68(2-3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- Anderson K. O., Bradley L. A., Young L. D., McDaniel L. K., Wise C. M. Rheumatoid arthritis: review of psychological factors related to etiology, effects, and treatment. Psychol Bull. 1985 Sep;98(2):358–387. [PubMed] [Google Scholar]
- BECK A. T., WARD C. H., MENDELSON M., MOCK J., ERBAUGH J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bandura A., O'Leary A., Taylor C. B., Gauthier J., Gossard D. Perceived self-efficacy and pain control: opioid and nonopioid mechanisms. J Pers Soc Psychol. 1987 Sep;53(3):563–571. doi: 10.1037//0022-3514.53.3.563. [DOI] [PubMed] [Google Scholar]
- Barbas H., Pandya D. N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989 Aug 15;286(3):353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bradley L. A., Young L. D., Anderson K. O., Turner R. A., Agudelo C. A., McDaniel L. K., Pisko E. J., Semble E. L., Morgan T. M. Effects of psychological therapy on pain behavior of rheumatoid arthritis patients. Treatment outcome and six-month followup. Arthritis Rheum. 1987 Oct;30(10):1105–1114. doi: 10.1002/art.1780301004. [DOI] [PubMed] [Google Scholar]
- Bushnell M. C., Duncan G. H. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78(2):415–418. doi: 10.1007/BF00228914. [DOI] [PubMed] [Google Scholar]
- Cervero F., Schaible H. G., Schmidt R. F. Tonic descending inhibition of spinal cord neurones driven by joint afferents in normal cats and in cats with an inflamed knee joint. Exp Brain Res. 1991;83(3):675–678. doi: 10.1007/BF00229846. [DOI] [PubMed] [Google Scholar]
- De Broucker T., Cesaro P., Willer J. C., Le Bars D. Diffuse noxious inhibitory controls in man. Involvement of the spinoreticular tract. Brain. 1990 Aug;113(Pt 4):1223–1234. doi: 10.1093/brain/113.4.1223. [DOI] [PubMed] [Google Scholar]
- Derbyshire S. W., Jones A. K., Devani P., Friston K. J., Feinmann C., Harris M., Pearce S., Watson J. D., Frackowiak R. S. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry. 1994 Oct;57(10):1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Morrell M. J., Vogt B. A. Contributions of anterior cingulate cortex to behaviour. Brain. 1995 Feb;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dias R., Robbins T. W., Roberts A. C. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996 Mar 7;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- Edwards L. C., Pearce S. A., Turner-Stokes L., Jones A. The Pain Beliefs Questionnaire: an investigation of beliefs in the causes and consequences of pain. Pain. 1992 Dec;51(3):267–272. doi: 10.1016/0304-3959(92)90209-T. [DOI] [PubMed] [Google Scholar]
- FOLTZ E. L., WHITE L. E., Jr Pain "relief" by frontal cingulumotomy. J Neurosurg. 1962 Feb;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- Fishbain D. A., Trescott J., Cutler B., Abdel-Moty E., Rosomoff R. S., Rosomoff H. L. Do some chronic pain patients with atypical facial pain overvalue and obsess about their pain? Psychosomatics. 1993 Jul-Aug;34(4):355–359. doi: 10.1016/S0033-3182(93)71870-8. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Frackowiak R. S. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991 Jul;11(4):690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Frith C. D., Friston K., Liddle P. F., Frackowiak R. S. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci. 1991 Jun 22;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- George M. S., Ketter T. A., Parekh P. I., Horwitz B., Herscovitch P., Post R. M. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995 Mar;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Hsieh J. C., Belfrage M., Stone-Elander S., Hansson P., Ingvar M. Central representation of chronic ongoing neuropathic pain studied by positron emission tomography. Pain. 1995 Nov;63(2):225–236. doi: 10.1016/0304-3959(95)00048-W. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Brown W. D., Friston K. J., Qi L. Y., Frackowiak R. S. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc Biol Sci. 1991 Apr 22;244(1309):39–44. doi: 10.1098/rspb.1991.0048. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Cunningham V. J., Ha-Kawa S., Fujiwara T., Luthra S. K., Silva S., Derbyshire S., Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994 Oct;33(10):909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Derbyshire S. W. Cerebral mechanisms operating in the presence and absence of inflammatory pain. Ann Rheum Dis. 1996 Jul;55(7):411–420. doi: 10.1136/ard.55.7.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. K., Friston K. J., Qi L. Y., Harris M., Cunningham V. J., Jones T., Feinman C., Frackowiak R. S. Sites of action of morphine in the brain. Lancet. 1991 Sep 28;338(8770):825–825. doi: 10.1016/0140-6736(91)90717-4. [DOI] [PubMed] [Google Scholar]
- Jones A. K., Friston K., Frackowiak R. S. Localization of responses to pain in human cerebral cortex. Science. 1992 Jan 10;255(5041):215–216. doi: 10.1126/science.1553549. [DOI] [PubMed] [Google Scholar]
- Kazis L. E., Meenan R. F., Anderson J. J. Pain in the rheumatic diseases. Investigation of a key health status component. Arthritis Rheum. 1983 Aug;26(8):1017–1022. doi: 10.1002/art.1780260811. [DOI] [PubMed] [Google Scholar]
- Keefe F. J., Affleck G., Lefebvre J. C., Starr K., Caldwell D. S., Tennen H. Pain coping strategies and coping efficacy in rheumatoid arthritis: a daily process analysis. Pain. 1997 Jan;69(1-2):35–42. doi: 10.1016/s0304-3959(96)03246-0. [DOI] [PubMed] [Google Scholar]
- Keefe F. J., Caldwell D. S., Martinez S., Nunley J., Beckham J., Williams D. A. Analyzing pain in rheumatoid arthritis patients. Pain coping strategies in patients who have had knee replacement surgery. Pain. 1991 Aug;46(2):153–160. doi: 10.1016/0304-3959(91)90070-E. [DOI] [PubMed] [Google Scholar]
- Kidd B. L., Morris V. H., Urban L. Pathophysiology of joint pain. Ann Rheum Dis. 1996 May;55(5):276–283. doi: 10.1136/ard.55.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit J., Kuypers H. G. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977 Sep 28;29(3-4):299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- König M., Zimmer A. M., Steiner H., Holmes P. V., Crawley J. N., Brownstein M. J., Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996 Oct 10;383(6600):535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- Lorig K. R., Mazonson P. D., Holman H. R. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum. 1993 Apr;36(4):439–446. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975 Sep;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- Pardo J. V., Pardo P. J., Janer K. W., Raichle M. E. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990 Jan;87(1):256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Gemba H. Electrical activity in the prefrontal cortex specific to no-go reaction of conditioned hand movement with colour discrimination in the monkey. Exp Brain Res. 1986;64(3):603–606. doi: 10.1007/BF00340499. [DOI] [PubMed] [Google Scholar]
- Sikes R. W., Vogt B. A. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992 Nov;68(5):1720–1732. doi: 10.1152/jn.1992.68.5.1720. [DOI] [PubMed] [Google Scholar]
- Talbot J. D., Marrett S., Evans A. C., Meyer E., Bushnell M. C., Duncan G. H. Multiple representations of pain in human cerebral cortex. Science. 1991 Mar 15;251(4999):1355–1358. doi: 10.1126/science.2003220. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T., Katayama Y., Ueno Y., Moriyasu N. Evidence for involvement of the frontal cortex in pain-related cerebral events in cats: increase in local cerebral blood flow by noxious stimuli. Brain Res. 1981 Jul 27;217(1):179–185. doi: 10.1016/0006-8993(81)90197-9. [DOI] [PubMed] [Google Scholar]
- Vaccarino A. L., Melzack R. Analgesia produced by injection of lidocaine into the anterior cingulum bundle of the rat. Pain. 1989 Nov;39(2):213–219. doi: 10.1016/0304-3959(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Derbyshire S., Jones A. K. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. Eur J Neurosci. 1996 Jul;8(7):1461–1473. doi: 10.1111/j.1460-9568.1996.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Vogt B. A., Rosene D. L., Pandya D. N. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979 Apr 13;204(4389):205–207. doi: 10.1126/science.107587. [DOI] [PubMed] [Google Scholar]
- Vogt L. J., Vogt B. A., Sikes R. W. Limbic thalamus in rabbit: architecture, projections to cingulate cortex and distribution of muscarinic acetylcholine, GABAA, and opioid receptors. J Comp Neurol. 1992 May 8;319(2):205–217. doi: 10.1002/cne.903190203. [DOI] [PubMed] [Google Scholar]