Abstract
OBJECTIVE—Analysis of the interaction of enteropathogenic bacteria with HLA B27 transfected murine fibroblasts showed a specific influence of HLA B27 on microbial invasiveness. This possible novel mechanism for the action of HLA B27 should be verified by using endogenous HLA B27 positive and negative human fibroblasts as a model for the direct interaction of arthritogenic bacteria and host cells. METHODS—Fibroblasts were obtained from healthy donors positive or negative for HLA B27; cultivated as monolayers; and infected with Salmonella enterica serovar enteritidis. RESULTS—Invasion and survival of bacteria in human cells was not influenced by the presence of HLA B27. Enhancement of HLA class I molecule expression by treatment of cultures with interferon gamma decreased invasion and survival of bacteria in both HLA B27 positive and negative cells. After disappearance of live bacteria lipopolysaccharide antigens persisted within cells. CONCLUSION—Endogenous HLA B27 does not modulate the direct interaction of Salmonella with human cells. Non-professional phagocytes are able to limit bacterial survival in cells, and interferon gamma accelerates killing of bacteria, but arthritogenic antigens persist after disappearance of live bacteria.
Full Text
The Full Text of this article is available as a PDF (143.5 KB).
Figure 1 .
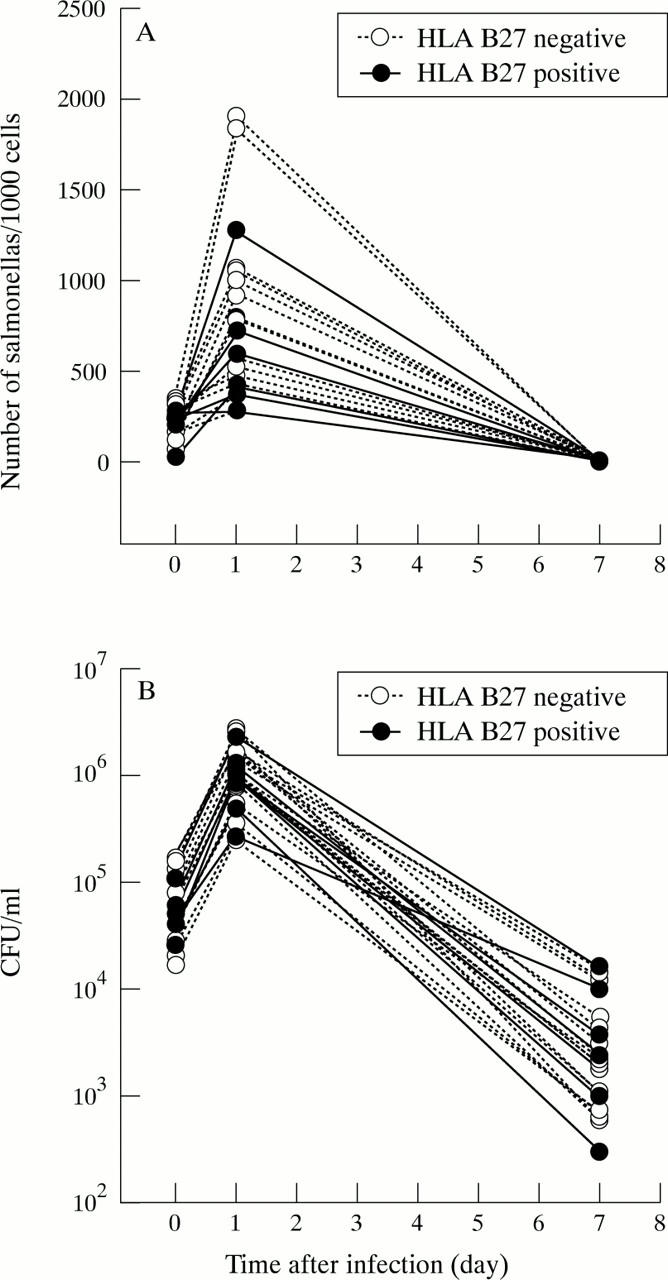
Influence of HLA B27 on invasion and survival of Salmonella enterica serotype enteritidis in 26 primary human fibroblast lines, six of which were HLA B27 positive and 20 HLA B27 negative. (A) Number of salmonellas per 100 cells after the infection (invasion) and one and seven days later (survival). Median (range) in HLA B27 positive/ negative cells were: day 0: 210 (30-268) / 251 (68-348); day 1: 505 (282-1280) / 794 (282-1910); day 7: 1 (1-6) / 2 (1-4). (B) Colony forming units per monolayer (CFU/ml) after the infection and one and seven days later. Median (range) in HLA B27 positive / negative cells were: day 0: 4.6 × 104 (2.6-11 × 104) / 7.4 × 104 (1.7-17 × 104); day 1: 9.5 × 105 (2.7-23 × 105) / 12.0 × 105 (2.5-27 × 105); day 7: 30 × 102 (3.1-160 × 102) / 22 × 102 (6.0 -150 × 102). Comparing the groups of HLA B27 positive and negative cell lines for the number of salmonellas per 100 cells and the CFU/ml at the three time points by U test did not yield any significant differences.
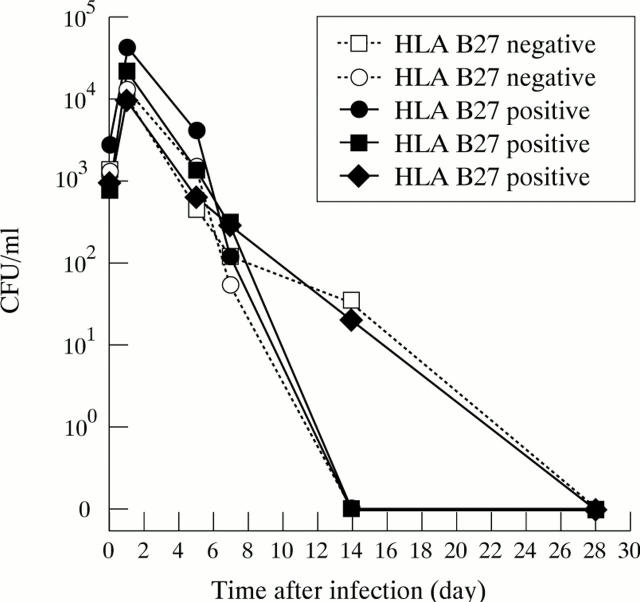
Figure 2 .
Persistent infection of Salmonella enterica serotype enteritidis in primary human fibroblasts. Three cell lines are HLA B27 positive, two lines HLA B27 negative.
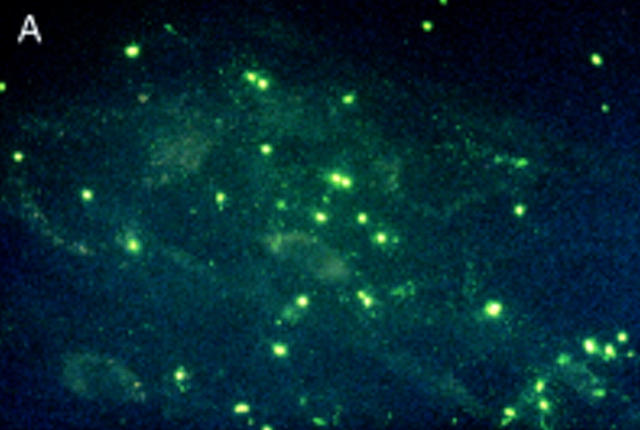
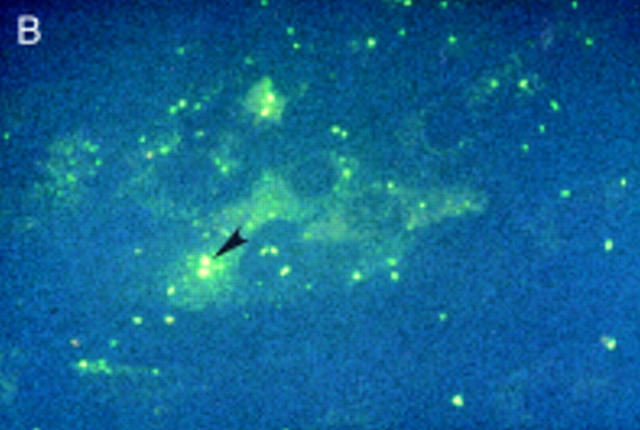
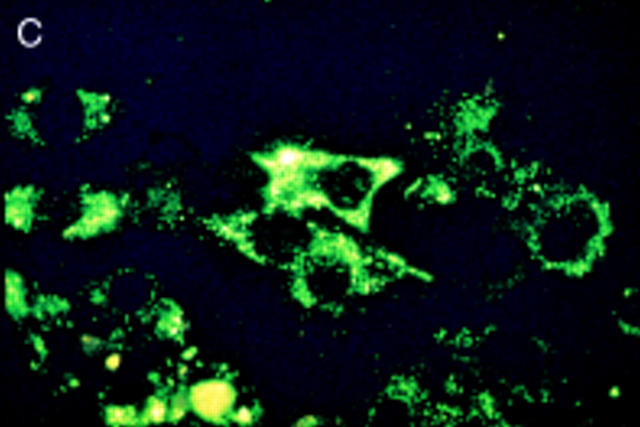
Figure 3 .
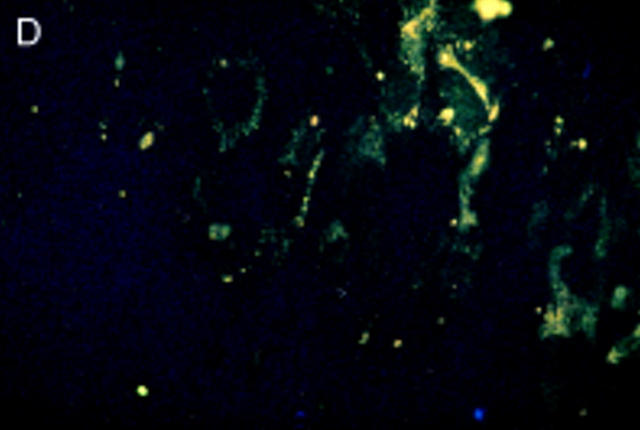
Salmonella antigen in primary human fibroblasts by indirect immunofluorescence staining for Salmonella lipopolysaccharide antigens directly after infection and two hours of antibiotic treatment (A) and seven (B) and 21 days later (C). Initially intact bacterial rods prevail. After seven days aggregates of cytoplasmic antigen deposits can be seen (arrowhead). After 21 days, at a time when the culture had become sterile, bacterial rods can no longer be identified, but finely dispersed antigen deposit can be seen in the cells. For control a parallel culture was stained with normal rabbit serum after 21 days of infection (D); to show cell morphology exposure time was doubled in comparison with fig 3C. Staining with the normal rabbit serum shows no green colour, but only faint yellow non-specific background staining.
Figure 4 .
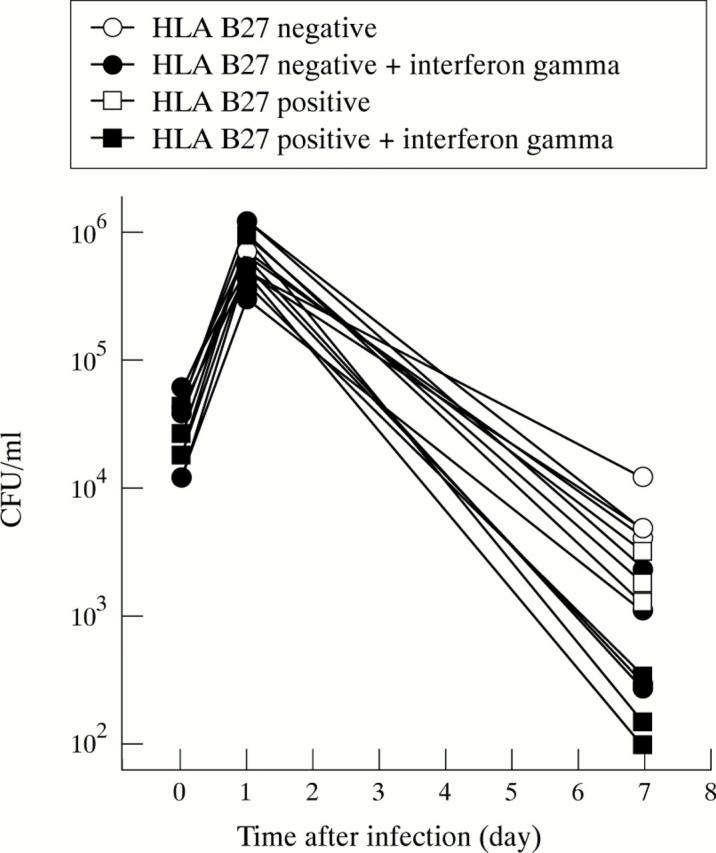
Effect of treatment with interferon gamma on the survival of Salmonella in primary human fibroblasts. Seven cell lines were infected with Salmonella enterica and then treated with 1000 IU/ml of interferon gamma or left untreated. The three HLA B27 positive cell lines are displayed with square symbols, the four HLA B27 negative cell lines with round symbols. Using ANOVA to compare treated and untreated cells treatment effect (p<0.001) and interaction (p<0.0001) were significantly different.
Figure 5 .
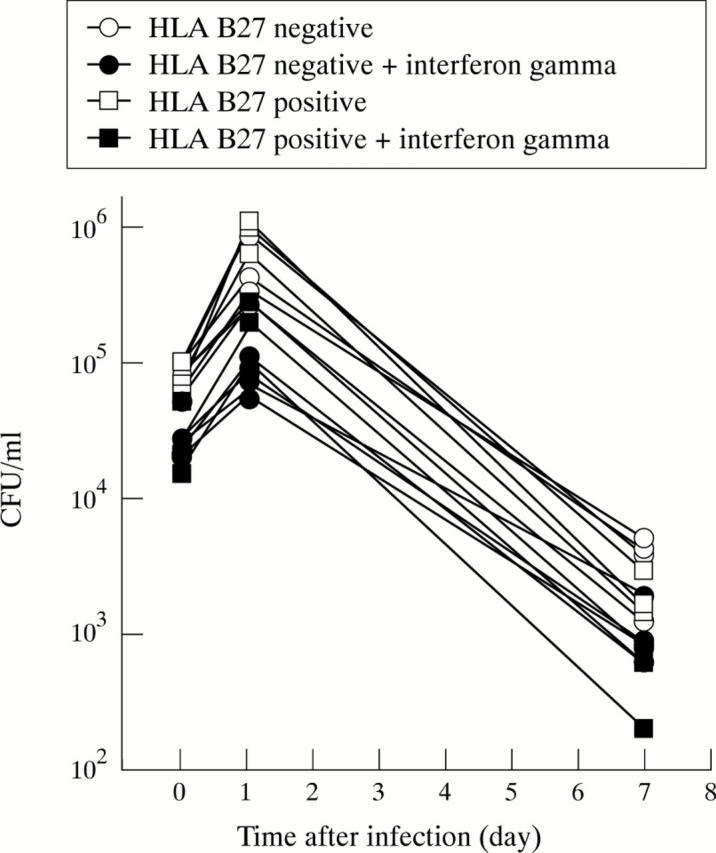
Effect of pre-treatment with interferon gamma on the invasion of Salmonella in primary human fibroblasts. Seven cell lines were treated with 1000 IU/ml of interferon gamma for 48 hours or left untreated and then infected with Salmonella enterica. The three HLA B27 positive cell lines are displayed with square symbols, the four HLA B27 negative cell lines with round symbols. Using ANOVA to compare treated and untreated cells treatment effect was highly significant (p<0.000005), but interaction was not, confirming the significant influence of interferon gamma on invasion.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galán J. E. Interactions of bacteria with non-phagocytic cells. Curr Opin Immunol. 1994 Aug;6(4):590–595. doi: 10.1016/0952-7915(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F., Finlay B. B. The varied lifestyles of intracellular pathogens within eukaryotic vacuolar compartments. Trends Microbiol. 1995 Oct;3(10):373–380. doi: 10.1016/s0966-842x(00)88982-9. [DOI] [PubMed] [Google Scholar]
- Garcia-del Portillo F., Zwick M. B., Leung K. Y., Finlay B. B. Salmonella induces the formation of filamentous structures containing lysosomal membrane glycoproteins in epithelial cells. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10544–10548. doi: 10.1073/pnas.90.22.10544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., Lindberg A. A., Mäki-Ikola O., von Essen R., Lahesmaa-Rantala R., Isomäki H., Saario R., Arnold W. J., Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990 Mar 24;335(8691):685–688. doi: 10.1016/0140-6736(90)90804-e. [DOI] [PubMed] [Google Scholar]
- Guilloteau L. A., Wallis T. S., Gautier A. V., MacIntyre S., Platt D. J., Lax A. J. The Salmonella virulence plasmid enhances Salmonella-induced lysis of macrophages and influences inflammatory responses. Infect Immun. 1996 Aug;64(8):3385–3393. doi: 10.1128/iai.64.8.3385-3393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B., Poole S., Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996 Jun;60(2):316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. A., Keat A. C. Reiter's syndrome and reactive arthritis: a current view. Semin Arthritis Rheum. 1994 Dec;24(3):190–210. doi: 10.1016/0049-0172(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Huppertz H. I., Chantler J. K. Restricted mumps virus infection of cells derived from normal human joint tissue. J Gen Virol. 1991 Feb;72(Pt 2):339–347. doi: 10.1099/0022-1317-72-2-339. [DOI] [PubMed] [Google Scholar]
- Huppertz H. I., Heesemann J. Experimental Yersinia infection of human synovial cells: persistence of live bacteria and generation of bacterial antigen deposits including "ghosts," nucleic acid-free bacterial rods. Infect Immun. 1996 Apr;64(4):1484–1487. doi: 10.1128/iai.64.4.1484-1487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz H. I., Heesemann J. The influence of HLA B27 and interferon-gamma on the invasion and persistence of yersinia in primary human fibroblasts. Med Microbiol Immunol. 1996 Nov;185(3):163–170. doi: 10.1007/s004300050027. [DOI] [PubMed] [Google Scholar]
- Huppertz H. I., Karch H., Heesemann J. Diagnostic value of synovial fluid analysis in children with reactive arthritis. Rheumatol Int. 1995;15(4):167–170. doi: 10.1007/BF00301775. [DOI] [PubMed] [Google Scholar]
- Kapasi K., Inman R. D. HLA-B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992 Jun 1;148(11):3554–3559. [PubMed] [Google Scholar]
- Kapasi K., Inman R. D. ME1 epitope of HLA-B27 confers class I-mediated modulation of gram-negative bacterial invasion. J Immunol. 1994 Jul 15;153(2):833–840. [PubMed] [Google Scholar]
- Lindgren S. W., Stojiljkovic I., Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht H., Kihlström E., Lindström F. D. Reactive arthritis after Salmonella among medical doctors--study of an outbreak. J Rheumatol. 1993 May;20(5):845–848. [PubMed] [Google Scholar]
- Mattila L., Leirisalo-Repo M., Koskimies S., Granfors K., Siitonen A. Reactive arthritis following an outbreak of Salmonella infection in Finland. Br J Rheumatol. 1994 Dec;33(12):1136–1141. doi: 10.1093/rheumatology/33.12.1136. [DOI] [PubMed] [Google Scholar]
- Merilahti-Palo R., Söderström K. O., Lahesmaa-Rantala R., Granfors K., Toivanen A. Bacterial antigens in synovial biopsy specimens in yersinia triggered reactive arthritis. Ann Rheum Dis. 1991 Feb;50(2):87–90. doi: 10.1136/ard.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olerup O. HLA-B27 typing by a group-specific PCR amplification. Tissue Antigens. 1994 Apr;43(4):253–256. doi: 10.1111/j.1399-0039.1994.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Schlaak J., Hermann E., Ringhoffer M., Probst P., Gallati H., Meyer zum Büschenfelde K. H., Fleischer B. Predominance of Th1-type T cells in synovial fluid of patients with Yersinia-induced reactive arthritis. Eur J Immunol. 1992 Nov;22(11):2771–2776. doi: 10.1002/eji.1830221103. [DOI] [PubMed] [Google Scholar]
- Thomson G. T., DeRubeis D. A., Hodge M. A., Rajanayagam C., Inman R. D. Post-Salmonella reactive arthritis: late clinical sequelae in a point source cohort. Am J Med. 1995 Jan;98(1):13–21. doi: 10.1016/S0002-9343(99)80076-X. [DOI] [PubMed] [Google Scholar]
- Thomson G. T., Minenko A., Schroeder M. L. Host risk factors for the development of reactive arthritis: a family study. J Rheumatol. 1993 Aug;20(8):1350–1352. [PubMed] [Google Scholar]
- Wuorela M., Jalkanen S., Toivanen P., Granfors K. Yersinia lipopolysaccharide is modified by human monocytes. Infect Immun. 1993 Dec;61(12):5261–5270. doi: 10.1128/iai.61.12.5261-5270.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]