Abstract
OBJECTIVE—Muscles are essential components of our sensorimotor system that help maintain balance and perform a smooth gait, but it is unclear whether arthritic damage adversely affects muscle sensorimotor function. Quadriceps sensorimotor function in patients with knee osteoarthritis (OA) was investigated, and whether these changes were associated with impairment of functional performance. METHODS—Quadriceps strength, voluntary activation, and proprioceptive acuity (joint position sense acuity) were assessed in 103 patients with knee OA and compared with 25 healthy control subjects. In addition, their postural stability, objective functional performance (the aggregate time for four activities of daily living), and disabilities (Lequesne index) were also investigated. RESULTS—Compared with the control subjects, the patients with knee OA had weaker quadriceps (differences between group mean 100N, CI 136, 63N), poorer voluntary activation (20% CI 13, 25%) that was associated with quadriceps weakness, and impaired acuity of knee joint position sense (1.28°, CI 0.84, 1.73°). As a group the patients were more unstable (p=0.0017), disabled (10, CI 7, 11), and had poorer functional performance (19.6 seconds, CI 14.3, 24.9 seconds). The most important predictors of disability were objective functional performance and quadriceps strength. CONCLUSIONS—In patients with knee OA, articular damage may reduce quadriceps motoneurone excitability, which decreases voluntary quadriceps activation thus contributing to quadriceps weakness, and diminishes proprioceptive acuity. The arthrogenic impairment in quadriceps sensorimotor function and decreased postural stability was associated with reduced functional performance of the patients.
Full Text
The Full Text of this article is available as a PDF (149.0 KB).
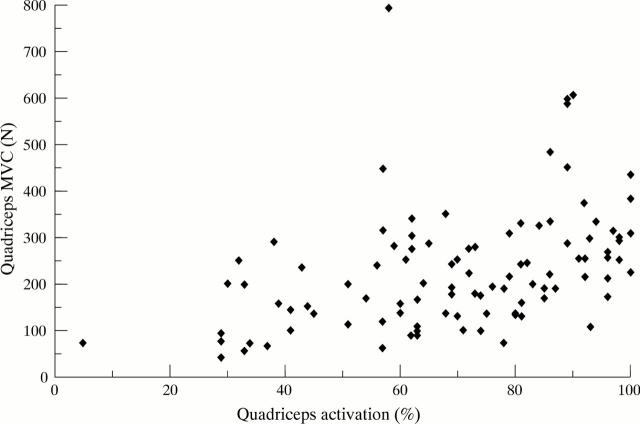
Figure 1 .
Scattergram of quadriceps strength and voluntary activation of patients with knee OA. Quadriceps strength was directly related to voluntary activation of the muscle (rs=0.486, p<0.001). MVC, maximum voluntary contraction. N, Newtons.
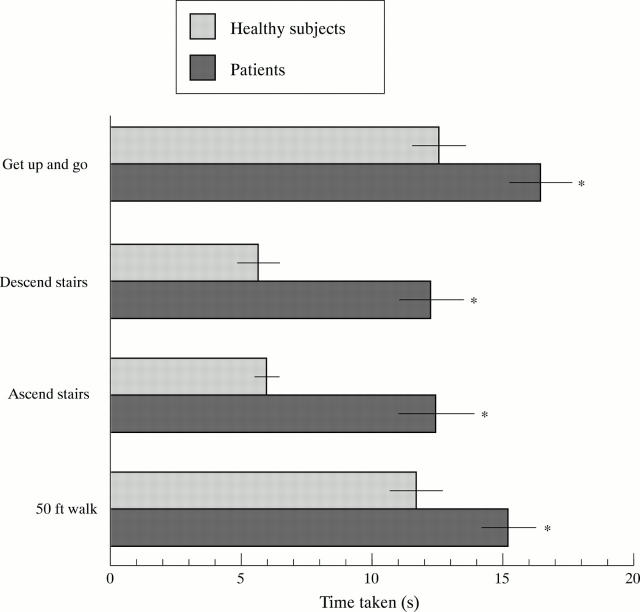
Figure 2 .
Time taken for the patients with knee OA and healthy subjects to perform the four common activities of daily living used to objectively assess functional performance. (Mean with 95% CI error bars). * Significantly slower (p<0.0001) than the healthy subjects.
Figure 3 .
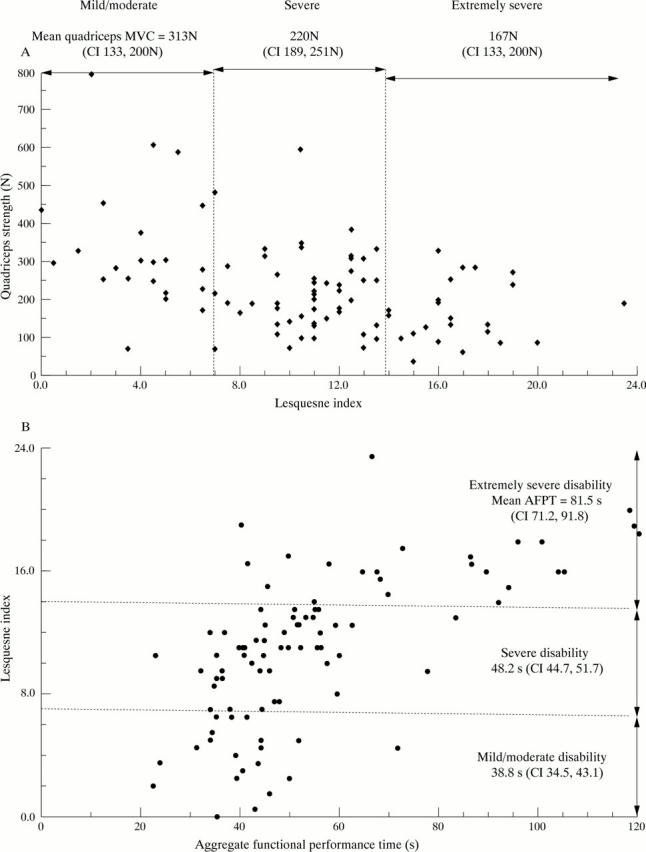
(A) Lequesne index and quadriceps strength of patients with knee OA. As quadriceps strength decreased the patient's disability increased (rs =−0.385, p<0.001). Mean quadriceps strength decreased in each arbitrarily defined category of disability (p<0.001). (B) Lequesne index and the aggregate functional performance time of the patients with knee OA. As the Lequesne index for disability increased the aggregate time of the tests increased (rs=0.665, p<0.001). The mean AFPT increased at each arbitrarily defined level of disability (p<0.001).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. M., Gandevia S. C., McKenzie D. K. Reliability of measurements of muscle strength and voluntary activation using twitch interpolation. Muscle Nerve. 1995 Jun;18(6):593–600. doi: 10.1002/mus.880180605. [DOI] [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson I., Eriksson E., Knutsson E., Arnér S. Reduction of pain inhibition on voluntary muscle activation by epidural analgesia. Orthopedics. 1986 Oct;9(10):1415–1419. doi: 10.3928/0147-7447-19861001-13. [DOI] [PubMed] [Google Scholar]
- Badley E. M., Rasooly I., Webster G. K. Relative importance of musculoskeletal disorders as a cause of chronic health problems, disability, and health care utilization: findings from the 1990 Ontario Health Survey. J Rheumatol. 1994 Mar;21(3):505–514. [PubMed] [Google Scholar]
- Barrett D. S., Cobb A. G., Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br. 1991 Jan;73(1):53–56. doi: 10.1302/0301-620X.73B1.1991775. [DOI] [PubMed] [Google Scholar]
- Claessens A. A., Schouten J. S., van den Ouweland F. A., Valkenburg H. A. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis. 1990 Oct;49(10):771–774. doi: 10.1136/ard.49.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnaghan J., Cooper C., Dieppe P., Kirwan J., McAlindon T., McCrae F. Clinical assessment of osteoarthritis of the knee. Ann Rheum Dis. 1990 Oct;49(10):768–770. doi: 10.1136/ard.49.10.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan P. W., Chandler J., Studenski S., Hughes M., Prescott B. How do physiological components of balance affect mobility in elderly men? Arch Phys Med Rehabil. 1993 Dec;74(12):1343–1349. doi: 10.1016/0003-9993(93)90090-w. [DOI] [PubMed] [Google Scholar]
- Ekdahl C., Andersson S. I. Standing balance in rheumatoid arthritis. A comparative study with healthy subjects. Scand J Rheumatol. 1989;18(1):33–42. doi: 10.3109/03009748909095401. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Era P., Heikkinen E. Postural sway during standing and unexpected disturbance of balance in random samples of men of different ages. J Gerontol. 1985 May;40(3):287–295. doi: 10.1093/geronj/40.3.287. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Baxendale R. H., Carnachan C., Hart I. K. The influence of joint afferent discharge on locomotion, proprioception and activity in conscious cats. Brain Res. 1985 Nov 11;347(1):41–48. doi: 10.1016/0006-8993(85)90887-x. [DOI] [PubMed] [Google Scholar]
- Fiatarone M. A., Marks E. C., Ryan N. D., Meredith C. N., Lipsitz L. A., Evans W. J. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990 Jun 13;263(22):3029–3034. [PubMed] [Google Scholar]
- Fisher N. M., Pendergast D. R., Gresham G. E., Calkins E. Muscle rehabilitation: its effect on muscular and functional performance of patients with knee osteoarthritis. Arch Phys Med Rehabil. 1991 May;72(6):367–374. [PubMed] [Google Scholar]
- Fitzpatrick R., McCloskey D. I. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994 Jul 1;478(Pt 1):173–186. doi: 10.1113/jphysiol.1994.sp020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M. A., Wyke B. Articular reflexes at the ankle joint: an electromyographic study of normal and abnormal influences of ankle-joint mechanoreceptors upon reflex activity in the leg muscles. Br J Surg. 1967 Dec;54(12):990–1001. doi: 10.1002/bjs.1800541204. [DOI] [PubMed] [Google Scholar]
- Gabriel S. E., Crowson C. S., Campion M. E., O'Fallon W. M. Indirect and nonmedical costs among people with rheumatoid arthritis and osteoarthritis compared with nonarthritic controls. J Rheumatol. 1997 Jan;24(1):43–48. [PubMed] [Google Scholar]
- Grace E. M., Gerecz E. M., Kassam Y. B., Buchanan H. M., Buchanan W. W., Tugwell P. S. 50-foot walking time: a critical assessment of an outcome measure in clinical therapeutic trials of antirheumatic drugs. Br J Rheumatol. 1988 Oct;27(5):372–374. doi: 10.1093/rheumatology/27.5.372. [DOI] [PubMed] [Google Scholar]
- Grimby G., Saltin B. The ageing muscle. Clin Physiol. 1983 Jun;3(3):209–218. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Guidelines for the diagnosis, investigation and management of osteoarthritis of the hip and knee. Report of a Joint Working Group of the British Society for Rheumatology and the Research Unit of the Royal College of Physicians. J R Coll Physicians Lond. 1993 Oct;27(4):391–396. [PMC free article] [PubMed] [Google Scholar]
- Hughes M. A., Duncan P. W., Rose D. K., Chandler J. M., Studenski S. A. The relationship of postural sway to sensorimotor function, functional performance, and disability in the elderly. Arch Phys Med Rehabil. 1996 Jun;77(6):567–572. doi: 10.1016/s0003-9993(96)90296-8. [DOI] [PubMed] [Google Scholar]
- Hurley M. V., Newham D. J. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993 Feb;32(2):127–131. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- Jayson M. I., St Dixon A. J. Intra-articular pressure in rheumatoid arthritis of the knee. I. Pressure changes during passive joint distension. Ann Rheum Dis. 1970 May;29(3):261–265. doi: 10.1136/ard.29.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Sjölander P., Sojka P. A sensory role for the cruciate ligaments. Clin Orthop Relat Res. 1991 Jul;(268):161–178. [PubMed] [Google Scholar]
- Jones D. W., Jones D. A., Newham D. J. Chronic knee effusion and aspiration: the effect on quadriceps inhibition. Br J Rheumatol. 1987 Oct;26(5):370–374. doi: 10.1093/rheumatology/26.5.370. [DOI] [PubMed] [Google Scholar]
- Lankhorst G. J., Van de Stadt R. J., Van der Korst J. K. The relationships of functional capacity, pain, and isometric and isokinetic torque in osteoarthrosis of the knee. Scand J Rehabil Med. 1985;17(4):167–172. [PubMed] [Google Scholar]
- Lord S. R., Lloyd D. G., Li S. K. Sensori-motor function, gait patterns and falls in community-dwelling women. Age Ageing. 1996 Jul;25(4):292–299. doi: 10.1093/ageing/25.4.292. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol. 1978 Nov;284:327–343. doi: 10.1113/jphysiol.1978.sp012543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S., Nayak U. S., Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil. 1986 Jun;67(6):387–389. [PubMed] [Google Scholar]
- Matthews P. B. Proprioceptors and their contribution to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharmacol. 1988 Apr;66(4):430–438. doi: 10.1139/y88-073. [DOI] [PubMed] [Google Scholar]
- McAlindon T. E., Cooper C., Kirwan J. R., Dieppe P. A. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993 Apr;52(4):258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D. I. Kinesthetic sensibility. Physiol Rev. 1978 Oct;58(4):763–820. doi: 10.1152/physrev.1978.58.4.763. [DOI] [PubMed] [Google Scholar]
- McComas A. J., Kereshi S., Quinlan J. A method for detecting functional weakness. J Neurol Neurosurg Psychiatry. 1983 Mar;46(3):280–282. doi: 10.1136/jnnp.46.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. P., Duthie E. H., Jr, Gambert S. R., Sepic S. B., Mollinger L. A. Age-related differences in knee muscle strength in normal women. J Gerontol. 1985 May;40(3):275–280. doi: 10.1093/geronj/40.3.275. [DOI] [PubMed] [Google Scholar]
- O'Connor B. L., Palmoski M. J., Brandt K. D. Neurogenic acceleration of degenerative joint lesions. J Bone Joint Surg Am. 1985 Apr;67(4):562–572. [PubMed] [Google Scholar]
- O'Connor B. L., Visco D. M., Brandt K. D., Albrecht M., O'Connor A. B. Sensory nerves only temporarily protect the unstable canine knee joint from osteoarthritis. Evidence that sensory nerves reprogram the central nervous system after cruciate ligament transection. Arthritis Rheum. 1993 Aug;36(8):1154–1163. doi: 10.1002/art.1780360817. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Martin R. B., Burr D. B., Caterson B., Boyd R. D., Goodwin C. Effects of mechanical loading on the tissues of the rabbit knee. J Orthop Res. 1984;2(3):221–234. doi: 10.1002/jor.1100020303. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Yang K. H., Riegger C., Kish V. L., O'Connor J. J. Relationship between lower limb dynamics and knee joint pain. J Orthop Res. 1991 May;9(3):398–405. doi: 10.1002/jor.1100090312. [DOI] [PubMed] [Google Scholar]
- Rutherford O. M., Jones D. A., Newham D. J. Clinical and experimental application of the percutaneous twitch superimposition technique for the study of human muscle activation. J Neurol Neurosurg Psychiatry. 1986 Nov;49(11):1288–1291. doi: 10.1136/jnnp.49.11.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford O. M., Jones D. A. The relationship of muscle and bone loss and activity levels with age in women. Age Ageing. 1992 Jul;21(4):286–293. doi: 10.1093/ageing/21.4.286. [DOI] [PubMed] [Google Scholar]
- Schaible H. G., Neugebauer V., Cervero F., Schmidt R. F. Changes in tonic descending inhibition of spinal neurons with articular input during the development of acute arthritis in the cat. J Neurophysiol. 1991 Sep;66(3):1021–1032. doi: 10.1152/jn.1991.66.3.1021. [DOI] [PubMed] [Google Scholar]
- Shakespeare D. T., Stokes M., Sherman K. P., Young A. Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clin Physiol. 1985 Apr;5(2):137–144. doi: 10.1111/j.1475-097x.1985.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Spencer J. D., Hayes K. C., Alexander I. J. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984 Apr;65(4):171–177. [PubMed] [Google Scholar]
- Wegener L., Kisner C., Nichols D. Static and dynamic balance responses in persons with bilateral knee osteoarthritis. J Orthop Sports Phys Ther. 1997 Jan;25(1):13–18. doi: 10.2519/jospt.1997.25.1.13. [DOI] [PubMed] [Google Scholar]
- Young A., Stokes M., Crowe M. Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest. 1984 Aug;14(4):282–287. doi: 10.1111/j.1365-2362.1984.tb01182.x. [DOI] [PubMed] [Google Scholar]