Abstract
OBJECTIVE—The aim of this study was to investigate in situ the expression of the classic vitronectin (VN) receptor consisting of the αv and β3 subunits in synovial lining cells (SLC) of chronic synovitis occurring in osteoarthritis (OA) and in rheumatoid arthritis (RA). The expression and function of αv and β3 as VN receptor in cultured fibroblast-like synoviocytes (FBS) derived from patients with OA and RA was also compared. METHODS—Expression of αv and β3 was examined immunohistochemically in normal synovial tissue and in synovial tisssue from patients with OA and RA. The effect of proinflammatory cytokines and of a synovial fluid of a patient with RA on the expression of the αv and β3 subunits of cultured FBS was determined by flow cytometry. Binding of OA and RA-FBS to VN was quantified using adhesion assays and the effect of interleukin 1β (IL1β) and tumour necrosis factor α (TNFα) on adhesion was measured. The specifity of the adhesion was tested by inhibition studies using monoclonal antibodies to integrin subunits. RESULTS—In in situ studies normal SLC showed a parallel distribution of αv and β3 subunits. OA-SLC strongly and uniformly expressed αv whereas RA-SLC showed heterogeneous expression of αv. In situ both OA-SLC and RA-SLC lacked the expression of the integrin subunit β3. In in vitro studies, OA-FBS and RA-FBS did not differ as regards expression of αv and β3, and VN attachment. Binding of RA-FBS to VN was partially blocked by antibodies against αv, β1, and β3 subunits, whereas only antibodies against αv and β3 inhibited the binding of OA-FBS to VN. The proinflammatory cytokines TNFα and IL1β increased the expression of αv and β3, and the VN binding of OA-FBS, whereas αv and β3 expression, and VN binding were downregulated in RA-FBS. Similar effects were found when the synovial fluid of an RA patient was used. CONCLUSION—The integrin subunit β3 seems to be one partner but not the major one with which the subunit αv forms functional vitronectin receptors in OA-FBS and RA-FBS. The interaction between synovial cells and inflammatory cytokines seems to be different for OA and RA; the basis for this difference, however, remains to be established.
Full Text
The Full Text of this article is available as a PDF (220.3 KB).
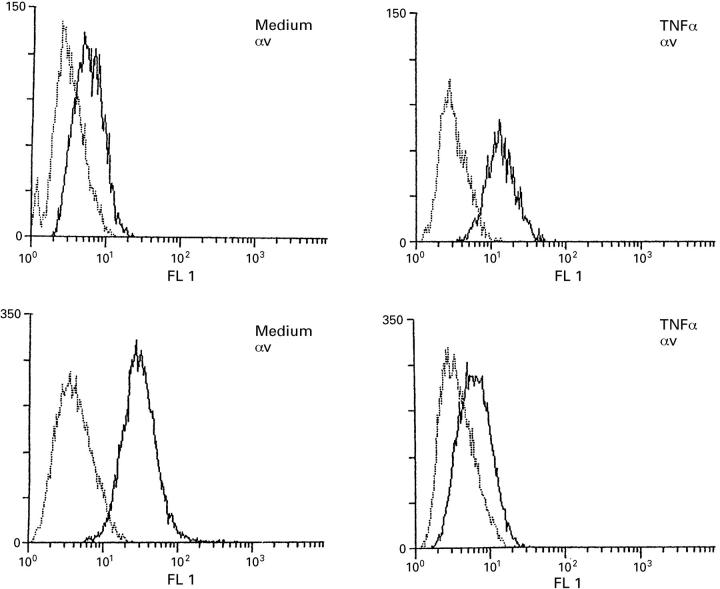
Figure 2 .
Histograms of flow cytometric analysis for the αv integrin subunit on FBS from OA (upper panels) and from RA (lower panels). The fluorescence obtained with isotypic negative control antibodies is plotted as a plain line, the results with anti-αv as a bold line.
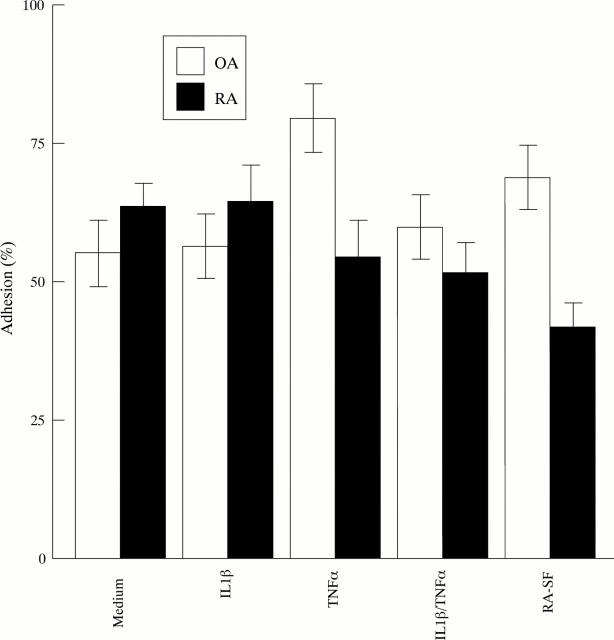
Figure 3 .
Effect of cytokines and synovial fluid (SF) from an arthritic joint of a RA patient on the binding of FBS cultures derived from two OA patients and from two RA patients, as determined by adhesion assay.
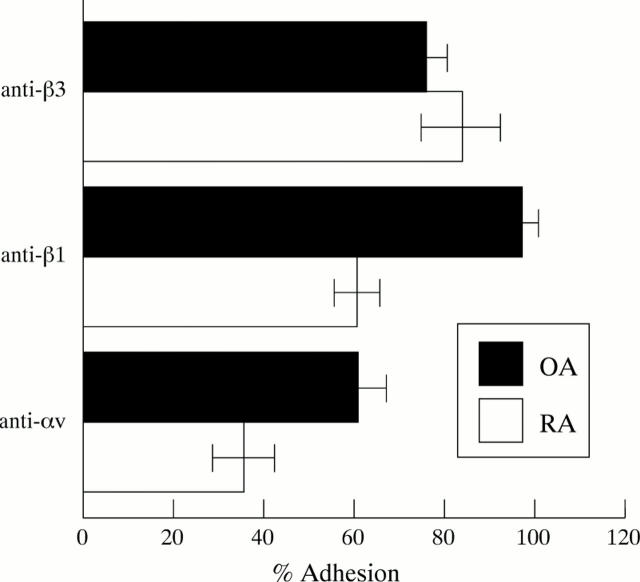
Figure 4 .
Inhibition of adhesion of FBS from OA and RA to VN. Monoclonal antibodies (mAb) against α3, α5, α6, αv, β1, and β3 were used; however only mAbs that blocked FBS binding are shown in the figure. Inhibition through the mAb is given as relative per cent adhesion compared with adhesion without antibodies (100%). Data represent the means of assays of three OA-FBS cultures and of three RA-FBS cultures.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aicher W. K., Heer A. H., Trabandt A., Bridges S. L., Jr, Schroeder H. W., Jr, Stransky G., Gay R. E., Eibel H., Peter H. H., Siebenlist U. Overexpression of zinc-finger transcription factor Z-225/Egr-1 in synoviocytes from rheumatoid arthritis patients. J Immunol. 1994 Jun 15;152(12):5940–5948. [PubMed] [Google Scholar]
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barnes D. W., Silnutzer J. Isolation of human serum spreading factor. J Biol Chem. 1983 Oct 25;258(20):12548–12552. [PubMed] [Google Scholar]
- Biesecker G. The complement SC5b-9 complex mediates cell adhesion through a vitronectin receptor. J Immunol. 1990 Jul 1;145(1):209–214. [PubMed] [Google Scholar]
- Defilippi P., van Hinsbergh V., Bertolotto A., Rossino P., Silengo L., Tarone G. Differential distribution and modulation of expression of alpha 1/beta 1 integrin on human endothelial cells. J Cell Biol. 1991 Aug;114(4):855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaziere A., Athanasou N. A. Adhesion receptors of intimal and subintimal cells of the normal synovial membrane. J Pathol. 1992 Oct;168(2):209–215. doi: 10.1002/path.1711680209. [DOI] [PubMed] [Google Scholar]
- Goto M., Okamoto M., Sasano M., Yanagisawa S., Miyamoto T., Nishioka K., Nakamura K., Aotuka S., Kawakami N., Yokohari R. Adherent synovial cells from nonrheumatoid arthritis do not release interleukin 1 beta and prostaglandin E2 spontaneously in longterm culture. J Rheumatol. 1990 Oct;17(10):1299–1302. [PubMed] [Google Scholar]
- Hayashi K., Madri J. A., Yurchenco P. D. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: an adhesion modulated by glycosaminoglycan. J Cell Biol. 1992 Nov;119(4):945–959. doi: 10.1083/jcb.119.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetland G., Pettersen H. B., Mollnes T. E., Johnson E. S-protein is synthesized by human monocytes and macrophages in vitro. Scand J Immunol. 1989 Jan;29(1):15–21. doi: 10.1111/j.1365-3083.1989.tb01094.x. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jahn B., Von Kempis J., Krämer K. L., Filsinger S., Hänsch G. M. Interaction of the terminal complement components C5b-9 with synovial fibroblasts: binding to the membrane surface leads to increased levels in collagenase-specific mRNA. Immunology. 1993 Feb;78(2):329–334. [PMC free article] [PubMed] [Google Scholar]
- Klein S., Giancotti F. G., Presta M., Albelda S. M., Buck C. A., Rifkin D. B. Basic fibroblast growth factor modulates integrin expression in microvascular endothelial cells. Mol Biol Cell. 1993 Oct;4(10):973–982. doi: 10.1091/mbc.4.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. J Immunol Methods. 1984 Mar 16;67(2):379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- Milis L., Morris C. A., Sheehan M. C., Charlesworth J. A., Pussell B. A. Vitronectin-mediated inhibition of complement: evidence for different binding sites for C5b-7 and C9. Clin Exp Immunol. 1993 Apr;92(1):114–119. doi: 10.1111/j.1365-2249.1993.tb05956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkari L., Aho H., Yli-Jama T., Larjava H., Jalkanen M., Heino J. Expression of integrin family of cell adhesion receptors in rheumatoid synovium. Alpha 6 integrin subunit in normal and hyperplastic synovial lining cell layer. Am J Pathol. 1993 Apr;142(4):1019–1027. [PMC free article] [PubMed] [Google Scholar]
- Nikkari L., Haapasalmi K., Aho H., Torvinen A., Sheppard D., Larjava H., Heino J. Localization of the alpha v subfamily of integrins and their putative ligands in synovial lining cell layer. J Rheumatol. 1995 Jan;22(1):16–23. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Reilly J. T., Nash J. R. Vitronectin (serum spreading factor): its localisation in normal and fibrotic tissue. J Clin Pathol. 1988 Dec;41(12):1269–1272. doi: 10.1136/jcp.41.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi N., Barth T., Henne C., Mechterscheimer G., Möller P. Synoviocytes in chronic synovitis in situ and cytokine stimulated synovial cells in vitro neo-express alpha 1, alpha 3 and alpha 5 chains of beta 1 integrins. Virchows Arch. 1994;425(2):171–180. doi: 10.1007/BF00230354. [DOI] [PubMed] [Google Scholar]
- Rinaldi N., Schwarz-Eywill M., Weis D., Leppelmann-Jansen P., Lukoschek M., Keilholz U., Barth T. F. Increased expression of integrins on fibroblast-like synoviocytes from rheumatoid arthritis in vitro correlates with enhanced binding to extracellular matrix proteins. Ann Rheum Dis. 1997 Jan;56(1):45–51. doi: 10.1136/ard.56.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney M., Condell D., Quinlan W., Daly L., Whelan A., Feighery C., Bresnihan B. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988 Aug;31(8):956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Trabandt A., Aicher W. K., Gay R. E., Sukhatme V. P., Fassbender H. G., Gay S. Spontaneous expression of immediately-early response genes c-fos and egr-1 in collagenase-producing rheumatoid synovial fibroblasts. Rheumatol Int. 1992;12(2):53–59. doi: 10.1007/BF00300977. [DOI] [PubMed] [Google Scholar]