Abstract
OBJECTIVE—To test the hypothesis that enthesophyte formation and osteophyte growth are positively associated and to look for associations between bone formation at different sites on the skeleton so that a simple measure of bone formation could be derived. METHODS—Visual examination of 337 adult skeletons. All common sites of either enthesophyte or osteophyte formation were inspected by a single observer who graded bone formation at these sites on a 0-3 scale. The total score for each feature was divided by the number of sites examined to derive an enthesophyte and an osteophyte score. Cronbach's α and principal components analysis were used to identify groupings. RESULTS—Enthesophyte formation was associated with gender (M>F) and age. There was a positive correlation between enthesophytes and osteophytes (r = 0.65, 95% confidence interval, 0.58 to 0.71) which remained after correction for age and gender. Principal components analysis indicated four different groupings of enthesophyte formation. By choosing one site from each group a simple index of total skeletal bone formation could be derived. CONCLUSIONS—Osteophytes and enthesophytes are associated, such that a proportion of the population can be classified as "bone formers". Enthesophyte groupings provide some clues to aetiopathogenesis. Bone formation should be investigated as a possible determinant of the heterogeneity of outcome and of treatment responses in common musculoskeletal disorders.
Full Text
The Full Text of this article is available as a PDF (219.4 KB).
Figure 1 .
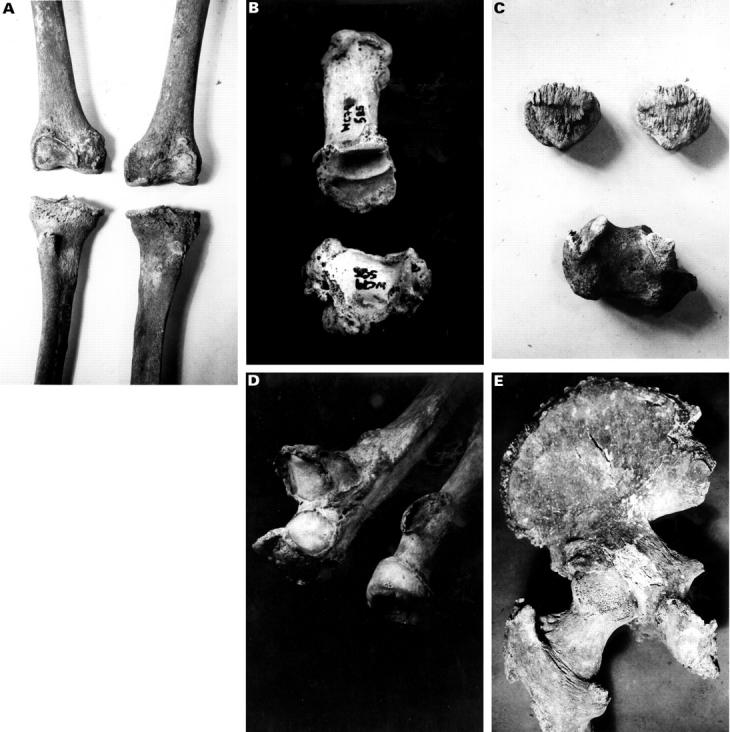
(A) Knees showing marked osteophytes grade III and enthesophytes at the tibial tubercle grade II. (B) Grade III osteophyte around the carpo-metacarpal joint of the thumb. (C) Calcaneum with grade II enthesophyte and patellae with grade III enthesophyte. (D) Radius with grade II enthesophyte at occipital protuberance and grade I osteophyte at radioulnar articulation. Ulna humeral joint has grade II osteophyte. (E) Hip joint showing greater trochanter of femur with grade II enthesophyte and lesser trochanter with grade I enthesophyte. The iliac crest also has enthesophytes, grade I, and the ischial tuberosity enthesophytes grade II.
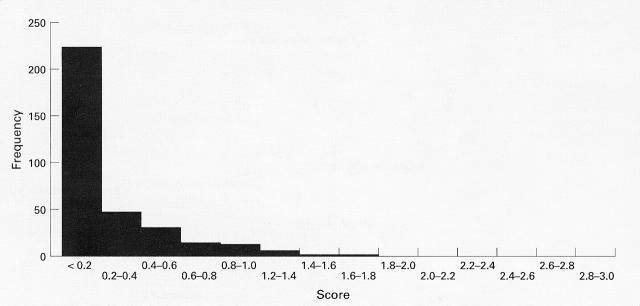
Figure 2 .
Histogram of the frequencies of skeletons in each category of osteophyte score. Each category has a range of 0.2 units. A positive skew is clearly evident
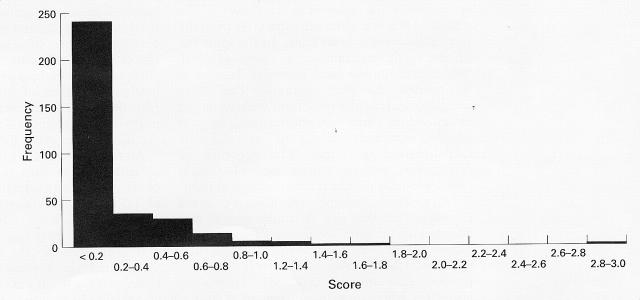
Figure 3 .
Histogram of the frequencies of skeletons in each category of enthesophyte score. Each category has a range of 0.2 units. A positive skew is clearly evident.
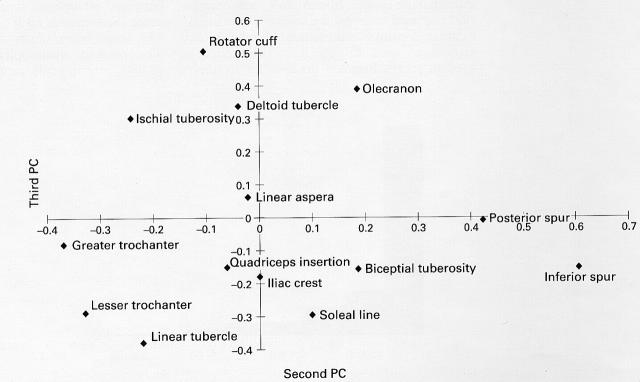
Figure 4 .
The loadings from each of the ligament insertion sites for the second and third principal components. This plot has been used to informally identify four groups of sites.
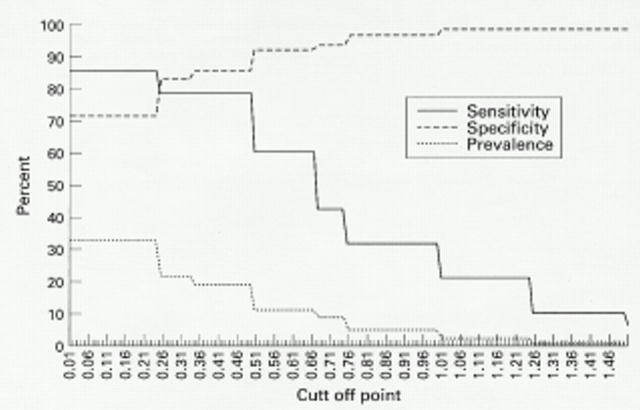
Figure 5 .
Characteristics of different "bone former" definitions—agreement with occurrence of DISH and prevalence.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FORESTIER J., ROTES-QUEROL J. Senile ankylosing hyperostosis of the spine. Ann Rheum Dis. 1950 Dec;9(4):321–330. doi: 10.1136/ard.9.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernborg J., Nilsson B. E. The relationship between osteophytes in the knee joint, osteoarthritis and aging. Acta Orthop Scand. 1973;44(1):69–74. doi: 10.3109/17453677308988675. [DOI] [PubMed] [Google Scholar]
- Julkunen H., Heinonen O. P., Pyörälä K. Hyperostosis of the spine in an adult population. Its relation to hyperglycaemia and obesity. Ann Rheum Dis. 1971 Nov;30(6):605–612. doi: 10.1136/ard.30.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkunen H., Heinonen O. P., Pyörälä K. Hyperostosis of the spine in an adult population. Its relation to hyperglycaemia and obesity. Ann Rheum Dis. 1971 Nov;30(6):605–612. doi: 10.1136/ard.30.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz R. W., Goldberg V. M. Studies of osteophyte pathogenesis in experimentally induced osteoarthritis. J Rheumatol. 1987 Apr;14(2):311–320. [PubMed] [Google Scholar]
- Resnick D., Niwayama G. Entheses and enthesopathy. Anatomical, pathological, and radiological correlation. Radiology. 1983 Jan;146(1):1–9. doi: 10.1148/radiology.146.1.6849029. [DOI] [PubMed] [Google Scholar]
- Resnick D., Niwayama G. Radiographic and pathologic features of spinal involvement in diffuse idiopathic skeletal hyperostosis (DISH). Radiology. 1976 Jun;119(3):559–568. doi: 10.1148/119.3.559. [DOI] [PubMed] [Google Scholar]
- Resnick D., Shaul S. R., Robins J. M. Diffuse idiopathic skeletal hyperostosis (DISH): Forestier's disease with extraspinal manifestations. Radiology. 1975 Jun;115(3):513–524. doi: 10.1148/15.3.513. [DOI] [PubMed] [Google Scholar]
- Rogers J., Watt I., Dieppe P. Comparison of visual and radiographic detection of bony changes at the knee joint. BMJ. 1990 Feb 10;300(6721):367–368. doi: 10.1136/bmj.300.6721.367. [DOI] [PMC free article] [PubMed] [Google Scholar]