Abstract
OBJECTIVE—To better understand the characteristics of synoviocytes located in the rheumatoid arthritis (RA) pannus. METHODS—One cell line, termed PSC, was cloned from RA pannus lesions. Phenotypic analysis was done by contrast microscopy, indirect immunostaining, and safranin O staining. Transcription of several protooncogenes and matrix degrading enzymes was evaluated. The expression of mRNA for collagen II was detected by in situ hybridisation. The ability of anchorage independent growth was assessed by soft agarose culture. RESULTS—PSCs showed a high transcription of protooncogenes c-fos, c-myc and c-jun. They also expressed mRNA for matrix degrading enzymes, such as collagenase, cathepsin B, and cathepsin L. Anchorage independent growth assay demonstrated that PSCs formed colonies in soft agar culture. Phenotypic analysis showed that this fibroblast-like PSC was stained intensely with anti-vimentin and anti-fibroblast antibody. In situ reverse transcriptase assay showed that the cell line expressed type II collagen mRNA. CONCLUSION—Alternative fibroblast-like cells were identified in the pannus lesion of RA sharing properties of fibroblasts and chondrocytes. These findings suggest that this fibroblast-like cell derived from pannus lesions may contribute to the destruction of the cartilage in RA.
Full Text
The Full Text of this article is available as a PDF (142.6 KB).
Figure 1 .
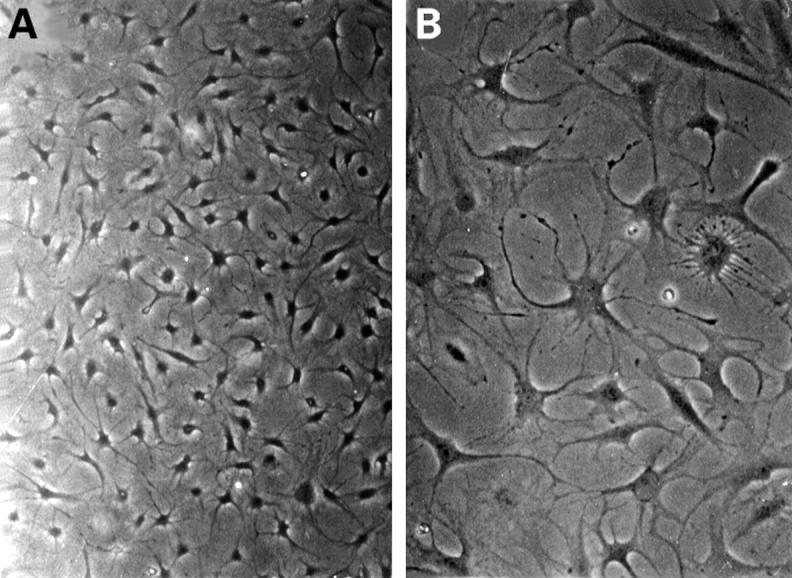
Contrast microscopy of cultured pannus synoviocytes. PSCs were cultured in a 90 mm dish. They were a homogeneous group of cells (A) and had many filopodia (B) (original magnification × 200).
Figure 2 .
Immunofluorescence micrograph of vimentin filaments in cultured PSCs.Under contrast microscope, almost all PSCs were stained with anti-vimentin antibody labelled with FITC. The network of intermediate filament vimentin was stained with antibody to vimentin protein. An extensive network of vimentin filament surrounds the nucleus and extends out to the cell periphery (fluorescence microscopy, original magnification × 400).
Figure 3 .
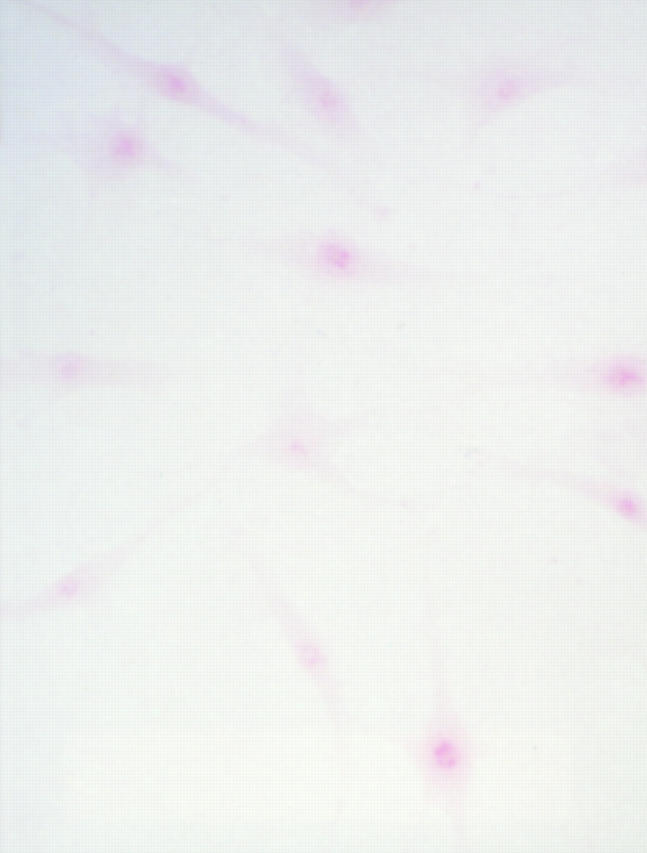
Safranin O staining of synoviocytes. Cloned cells were seeded into the slide chamber, and fixed three days later with acetone, then subjected to the staining procedure described in the text. PSCs stained with red colour represent positive staining (original magnification × 200).
Figure 4 .
Type II collagen in situ reverse transcription. In situ reverse transcriptase assay was performed on the slide chamber with cultured PSCs to evaluate the expression of type II collagen mRNA. PCR amplification was performed with digoxigenin labelled oligonucleotide probe and sequential immunostaining with sheep anti-digoxigenin conjugated rhodamine. Cells picked up from the soft agar highly expressed type II collagen mRNA (A). High power view shows one PSC with several filopodia. Type II collagen mRNA shows a scattered pattern (B) (confocal microscopy, (A) original magnification × 200, (B) × 400).
Figure 5 .
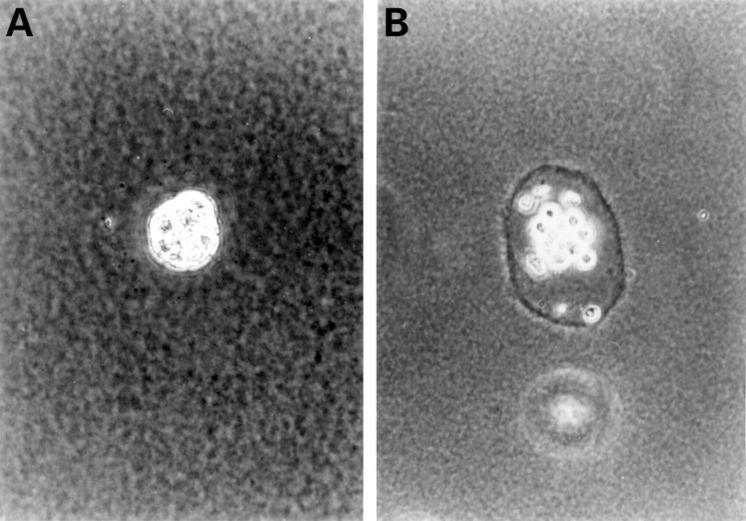
Colony growth in anchorage independent growth assay. After culture for four weeks, PSCs formed colonies in soft agarose (A). Under the phase contrast microscope, a number of the colonies have a ring formed in the extracellular matrix (B) (contrast microscopy, (A) and (B) original magnification × 200).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard S. A., Muirden K. D., Camplejohn K. L., Maini R. N. Chondrocyte-derived cells and matrix at the rheumatoid cartilage-pannus junction identified with monoclonal antibodies. Rheumatol Int. 1987;7(4):153–159. doi: 10.1007/BF00270363. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Conrad G. W., Hart G. W., Chen Y. Differences in vitro between fibroblast-like cells from cornea, heart, and skin of embryonic chicks. J Cell Sci. 1977 Aug;26:119–137. doi: 10.1242/jcs.26.1.119. [DOI] [PubMed] [Google Scholar]
- Cooke T. D. Rheumatoid arthritis pannus: true or false? Arthritis Rheum. 1985 Oct;28(10):1195–1198. doi: 10.1002/art.1780281021. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G. Histomorphological basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983 Mar;3(2):141–155. doi: 10.1016/s0174-173x(83)80040-5. [DOI] [PubMed] [Google Scholar]
- Fassbender H. G., Simmling-Annefeld M., Stofft E. Transformation der Synovialzellen bei rheumatoider Arthritis. Verh Dtsch Ges Pathol. 1980;64:193–212. [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Goto M., Sasano M., Yamanaka H., Miyasaka N., Kamatani N., Inoue K., Nishioka K., Miyamoto T. Spontaneous production of an interleukin 1-like factor by cloned rheumatoid synovial cells in long-term culture. J Clin Invest. 1987 Sep;80(3):786–796. doi: 10.1172/JCI113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A. Hypothesis: in vitro evidence for the invasive and tumor-like properties of the rheumatoid pannus. J Rheumatol. 1983 Dec;10(6):845–851. [PubMed] [Google Scholar]
- Hasunuma T., Nakajima T., Aono H., Sato K., Matsubara T., Yamamoto K., Nishioka K. Establishment and characterization of synovial cell clones with integrated human T-cell leukemia virus type-I. Clin Immunol Immunopathol. 1994 Jul;72(1):90–97. doi: 10.1006/clin.1994.1111. [DOI] [PubMed] [Google Scholar]
- Ishikawa K. Chondrocytes that accumulate proteoglycans and inorganic pyrophosphate in the pathogenesis of chondrocalcinosis. Arthritis Rheum. 1985 Jan;28(1):118–120. doi: 10.1002/art.1780280122. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Shimoyama M., Tobinai K., Ito M., Ito S., Ikeda S., Tajima K., Shimotohno K., Sugimura T. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5620–5624. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Johnell O., Hulth A., Holmdahl R., Rubin K. Reactivity of monoclonal anti-type II collagen antibodies with cartilage and synovial tissue in rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 1986 Jun;29(6):730–738. doi: 10.1002/art.1780290605. [DOI] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Lafyatis R., Remmers E. F., Roberts A. B., Yocum D. E., Sporn M. B., Wilder R. L. Anchorage-independent growth of synoviocytes from arthritic and normal joints. Stimulation by exogenous platelet-derived growth factor and inhibition by transforming growth factor-beta and retinoids. J Clin Invest. 1989 Apr;83(4):1267–1276. doi: 10.1172/JCI114011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciewicz R. A., Wotton S. F., Etherington D. J., Duance V. C. Susceptibility of the cartilage collagens types II, IX and XI to degradation by the cysteine proteinases, cathepsins B and L. FEBS Lett. 1990 Aug 20;269(1):189–193. doi: 10.1016/0014-5793(90)81151-d. [DOI] [PubMed] [Google Scholar]
- Muirden K. D. Electron microscopic studies of the synovial-cartilage junction in rheumatoid arthritis. Eur J Rheumatol Inflamm. 1982;5(1):30–38. [PubMed] [Google Scholar]
- Müller-Ladner U., Kriegsmann J., Tschopp J., Gay R. E., Gay S. Demonstration of granzyme A and perforin messenger RNA in the synovium of patients with rheumatoid arthritis. Arthritis Rheum. 1995 Apr;38(4):477–484. doi: 10.1002/art.1780380404. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Mort J. S., Roughley P. J. Cartilage proteoglycan aggregate is degraded more extensively by cathepsin L than by cathepsin B. Biochem J. 1990 Mar 1;266(2):569–573. [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Gay R., Gay S., Miller E. J. Transitions in collagen types during matrix-induced cartilage, bone, and bone marrow formation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5589–5592. doi: 10.1073/pnas.74.12.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Shapiro F., Koide S., Glimcher M. J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993 Apr;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Morphologic observations in the early phase of the cartilage-pannus junction. Light and electron microscopic studies of active cellular pannus. Arthritis Rheum. 1983 Apr;26(4):472–478. doi: 10.1002/art.1780260404. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Tanaka Y., Fujita T., Tokuhisa T. Destructive arthritis without lymphocyte infiltration in H2-c-fos transgenic mice. J Immunol. 1992 May 15;148(10):3100–3104. [PubMed] [Google Scholar]
- Shiozawa S., Tokuhisa T. Contribution of synovial mesenchymal cells to the pathogenesis of rheumatoid arthritis. Semin Arthritis Rheum. 1992 Feb;21(4):267–273. doi: 10.1016/0049-0172(92)90058-l. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. doi: 10.1146/annurev.bi.57.070188.003113. [DOI] [PubMed] [Google Scholar]
- Torry D. J., Richards C. D., Podor T. J., Gauldie J. Anchorage-independent colony growth of pulmonary fibroblasts derived from fibrotic human lung tissue. J Clin Invest. 1994 Apr;93(4):1525–1532. doi: 10.1172/JCI117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabandt A., Gay R. E., Fassbender H. G., Gay S. Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1444–1451. doi: 10.1002/art.1780341116. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Yagi K., Ohya Y., Kimata K. Scleral fibroblasts of the chick embryo differentiate into chondrocytes in soft-agar culture. In Vitro Cell Dev Biol. 1992 Sep-Oct;28A(9-10):603–608. doi: 10.1007/BF02631034. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J., Firestein G. S. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994 Jun;37(6):783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]