Abstract
OBJECTIVE—The capacity of articular cartilage matrix to recover during 50 weeks of remobilisation after an atrophy caused by 11 weeks of immobilisation of the knee (stifle) joint in 90° flexion starting at the age of 29 weeks, was studied in young beagle dogs. METHODS—Proteoglycan concentration (uronic acid) and synthesis ([35S]sulphate incorporation) were determined in six and three knee joint surface locations, respectively. Proteoglycans extracted from the cartilages were characterised by chemical determinations, gel filtration, and western blotting for chondroitin sulphate epitope 3B3. RESULTS—The proteoglycan concentrations that were reduced in all sample sites immediately after the immobilisation, remained 14-28% lower than controls after 50 weeks of remobilisation in the patella, the summit of medial femoral condyle, and the superior femoropatellar surface. In the contralateral joint, there was a 49% increase of proteoglycans in the inferior femoropatellar surface after remobilisation, while a 34% decrease was simultaneously noticed on the summit of the medial femoral condyle. Total proteoglycan synthesis was not significantly changed after immobilisation or 50 weeks' remobilisation in the treated or contralateral joint, compared with age matched controls. The chondroitin 6- to 4- sulphate ratio was reduced by immobilisation both in the radioactively labelled and the total tissue proteoglycans. In the remobilised joint, this ratio was restored in femur, while in tibia it remained at a level lower than controls. Neither immobilisation nor remobilisation induced epitopes recognised by the monoclonal antibody 3B3 on native (undigested) proteoglycans. CONCLUSION—These results show that the depletion of proteoglycans observed after 11 weeks of immobilisation was not completely restored in certain surface sites after 50 weeks of remobilisation. The significant changes that developed in the contralateral joint during the remobilisation period give further support to the idea that a permanent alteration of matrix metabolism results even from a temporary modification of loading pattern in immature joints.
Full Text
The Full Text of this article is available as a PDF (219.4 KB).
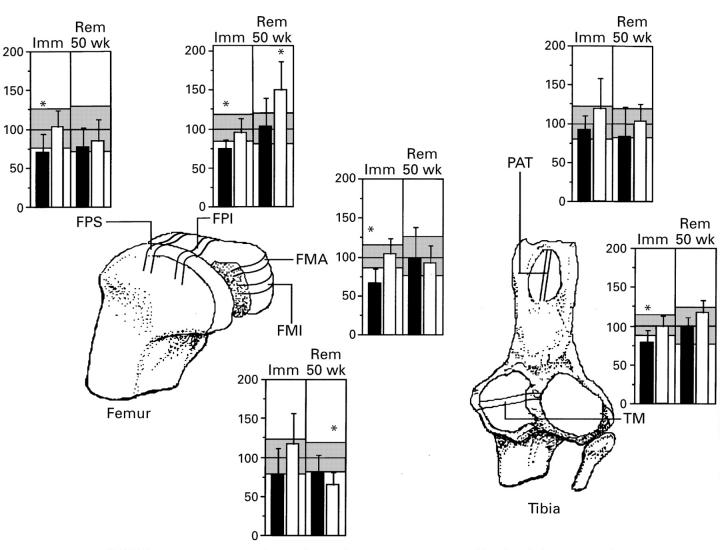
Figure 1 .
Concentration of extractable uronic acid in the articular cartilage of canine knee joints after 11 weeks of immobilisation (Imm), and after 50 weeks' remobilisation (Rem)of the limb that had been cast. The concentration of uronic acid was measured as nmol per mg wet weight, and the results were normalised to the level of littermate control animals (100% level shown in the figure). The shaded range in the background of each histogram indicates 1 SD from the mean of controls. Superior femoropatellar area, FPS; inferior femoropatellar area, FPI; anterior medial femoral condyle, FMA; intermediate section of the medial femoral condyle, FMI; patella, PAT; medial tibial condyle, TM; filled bars, immobilised or remobilised joint; open bars, contralateral joint. Asterisk shows significant changes (p≤0.05) compared with age matched control group.
Figure 2 .
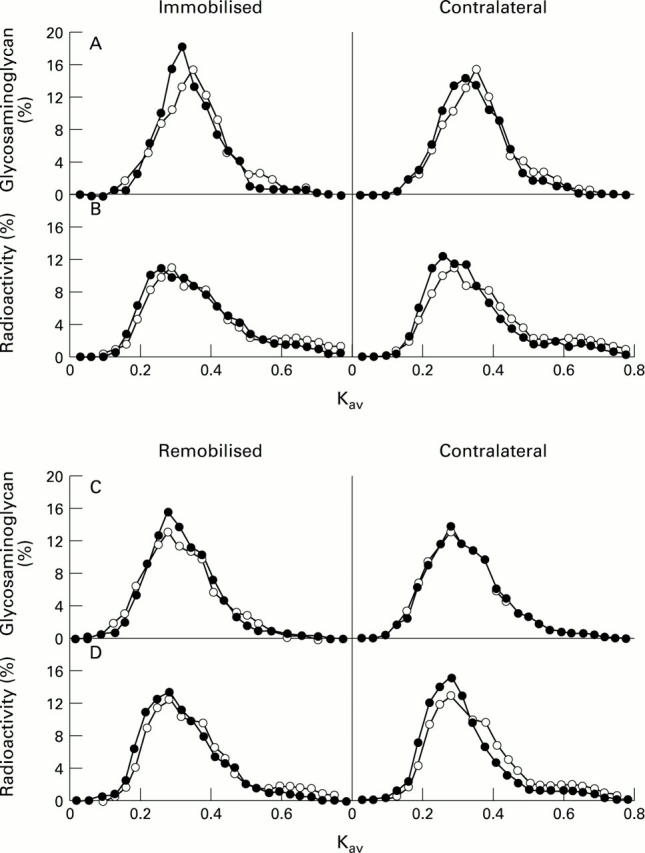
Immobilisation and remobilisation effects on proteoglycan size analysed with Sephacryl S-500 gel filtration. Pooled extracts from the immobilised anterior medial femoral condyle (FMA) and its contralateral joint (A and B, respectively), and remobilised and its contralateral joint (C and D, respectively) were chromatographed in the presence of 4 M GuCl, and the fractions were analysed for total proteoglycans (A and C) and for radioactivity (B and D). Each chromatography was repeated three times to confirm the changes in the profiles. Open symbols represent samples from the control group, and closed symbols those from the immobilised/remobilised limbs, treated and contralateral shown in separate panels.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens F., Kraft E. L., Oegema T. R., Jr Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J Orthop Res. 1989;7(3):335–343. doi: 10.1002/jor.1100070305. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bonassar L. J., Frank E. H., Murray J. C., Paguio C. G., Moore V. L., Lark M. W., Sandy J. D., Wu J. J., Eyre D. R., Grodzinsky A. J. Changes in cartilage composition and physical properties due to stromelysin degradation. Arthritis Rheum. 1995 Feb;38(2):173–183. doi: 10.1002/art.1780380205. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Caterson B., Baker J. R. Immunological determinants of proteoglycans. Antibodies against the unsaturated oligosaccharide products of chondroitinase ABC-digested cartilage proteoglycans. J Biol Chem. 1980 Aug 10;255(15):7102–7105. [PubMed] [Google Scholar]
- Eronen I., Videman T., Friman C., Michelsson J. E. Glycosaminoglycan metabolism in experimental osteoarthrosis caused by immobilization. Acta Orthop Scand. 1978 Aug;49(4):329–334. doi: 10.3109/17453677809050083. [DOI] [PubMed] [Google Scholar]
- Finsterbush A., Friedman B. Early changes in immobilized rabbits knee joints: a light and electron microscopic study. Clin Orthop Relat Res. 1973 May;(92):305–319. doi: 10.1097/00003086-197305000-00027. [DOI] [PubMed] [Google Scholar]
- Gibson T. J., Schumacher H. R., Pascual E., Brighton C. Arthropathy, skin and bone lesions in pancreatic disease. J Rheumatol. 1975 Mar;2(1):7–13. [PubMed] [Google Scholar]
- Jortikka M., Lammi M. J., Parkkinen J. J., Lahtinen R., Tammi M. I. A high sensitivity dot-blot assay for proteoglycans by cuprolinic blue precipitation. Connect Tissue Res. 1993;29(4):263–272. doi: 10.3109/03008209309016832. [DOI] [PubMed] [Google Scholar]
- Jurvelin J., Kiviranta I., Sämänen A. M., Tammi M., Helminen H. J. Partial restoration of immobilization-induced softening of canine articular cartilage after remobilization of the knee (stifle) joint. J Orthop Res. 1989;7(3):352–358. doi: 10.1002/jor.1100070307. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Jurvelin J., Tammi M., Sämänen A. M., Helminen H. J. Weight bearing controls glycosaminoglycan concentration and articular cartilage thickness in the knee joints of young beagle dogs. Arthritis Rheum. 1987 Jul;30(7):801–809. doi: 10.1002/art.1780300710. [DOI] [PubMed] [Google Scholar]
- Korver G. H., van de Stadt R. J., van Kampen G. P., Kiljan E., van der Korst J. K. Bovine sesamoid bones: a culture system for anatomically intact articular cartilage. In Vitro Cell Dev Biol. 1989 Dec;25(12):1099–1106. doi: 10.1007/BF02621260. [DOI] [PubMed] [Google Scholar]
- Kostenszky K. S., Oláh E. H. Effect of increased functional demand on the glucosaminoglycan (mucopolysaccharide) content of the articular cartilage. Acta Biol Acad Sci Hung. 1972;23(1):75–82. [PubMed] [Google Scholar]
- Käpä E., Holm S., Inkinen R., Lammi M. J., Tammi M., Vanharanta H. Proteoglycan chemistry in experimentally injured porcine intervertebral disk. J Spinal Disord. 1994 Aug;7(4):296–306. [PubMed] [Google Scholar]
- Lammi M. J., Häkkinen T. P., Parkkinen J. J., Hyttinen M. M., Jortikka M., Helminen H. J., Tammi M. I. Adaptation of canine femoral head articular cartilage to long distance running exercise in young beagles. Ann Rheum Dis. 1993 May;52(5):369–377. doi: 10.1136/ard.52.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammi M., Tammi M. Densitometric assay of nanogram quantities of proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal Biochem. 1988 Feb 1;168(2):352–357. doi: 10.1016/0003-2697(88)90329-6. [DOI] [PubMed] [Google Scholar]
- Langenskiöld A., Michelsson J. E., Videman T. Osteoarthritis of the knee in the rabbit produced by immobilization. Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop Scand. 1979 Feb;50(1):1–14. doi: 10.3109/17453677909024083. [DOI] [PubMed] [Google Scholar]
- Mourão P. A. Distribution of chondroitin 4-sulfate and chondroitin 6-sulfate in human articular and growth cartilage. Arthritis Rheum. 1988 Aug;31(8):1028–1033. doi: 10.1002/art.1780310814. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Ratcliffe A., Poole A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- Oláh E. H., Kostenszky K. S. Effect of altered functional demand on the glycosaminoglycan content of the articular cartilage of dogs. Acta Biol Acad Sci Hung. 1972;23(2):195–200. [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 1982 Oct;25(10):1201–1208. doi: 10.1002/art.1780251009. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Brandt K. D. Running inhibits the reversal of atrophic changes in canine knee cartilage after removal of a leg cast. Arthritis Rheum. 1981 Nov;24(11):1329–1337. doi: 10.1002/art.1780241101. [DOI] [PubMed] [Google Scholar]
- Palmoski M. J., Colyer R. A., Brandt K. D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980 Mar;23(3):325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- Palmoski M., Perricone E., Brandt K. D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979 May;22(5):508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- Paukkonen K., Jurvelin J., Helminen H. J. Effects of immobilization on the articular cartilage in young rabbits. A quantitative light microscopic stereological study. Clin Orthop Relat Res. 1986 May;(206):270–280. [PubMed] [Google Scholar]
- Ratcliffe A., Shurety W., Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993 Apr;36(4):543–551. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Simkin P. A., Brunzell J. D., Wisner D., Fiechtner J. J., Carlin J. S., Willkens R. F. Free fatty acids in the pancreatitic arthritis syndrome. Arthritis Rheum. 1983 Feb;26(2):127–132. doi: 10.1002/art.1780260202. [DOI] [PubMed] [Google Scholar]
- Sood S. C. A study of the effects of experimental immobilisation on rabbit articular cartilage. J Anat. 1971 Apr;108(Pt 3):497–507. [PMC free article] [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Jurvelin J., Kiviranta I., Helminen H. J. Proteoglycan alterations following immobilization and remobilization in the articular cartilage of young canine knee (stifle) joint. J Orthop Res. 1990 Nov;8(6):863–873. doi: 10.1002/jor.1100080612. [DOI] [PubMed] [Google Scholar]
- Sämänen A. M., Tammi M., Kiviranta I., Jurvelin J., Helminen H. J. Levels of chondroitin-6-sulfate and nonaggregating proteoglycans at articular cartilage contact sites in the knees of young dogs subjected to moderate running exercise. Arthritis Rheum. 1989 Oct;32(10):1282–1292. doi: 10.1002/anr.1780321014. [DOI] [PubMed] [Google Scholar]
- Tammi M., Sämänen A. M., Jauhiainen A., Malminen O., Kiviranta I., Helminen H. Proteoglycan alterations in rabbit knee articular cartilage following physical exercise and immobilization. Connect Tissue Res. 1983;11(1):45–55. doi: 10.3109/03008208309015010. [DOI] [PubMed] [Google Scholar]
- Videman T., Eronen I., Friman C. Glycosaminoglycan metabolism in experimental osteoarthritis caused by immobilization. The effects of different periods of immobilization and follow-up. Acta Orthop Scand. 1981 Feb;52(1):11–21. doi: 10.3109/17453678108991751. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Brandt K. D. Immobilization ameliorates chemically-induced articular cartilage damage. Arthritis Rheum. 1984 Feb;27(2):208–216. doi: 10.1002/art.1780270213. [DOI] [PubMed] [Google Scholar]