Abstract
OBJECTIVES—To determine if peptides containing the `shared epitope' sequence, QKRAA, from either endogenous, HLA-DRβ1(0401), or exogenous, Escherichia coli dnaJ, sources activate T cells in recent onset rheumatoid arthritis (RA). METHODS—Peripheral blood mononuclear cell (PBMC) proliferative and whole blood cytokine responses to shared epitope containing peptides from DRβ1(0401) and E coli dnaJ, to control peptides from DRβ1(0402) and hsp40 and to the recall antigen, tetanus toxoid, were tested in 20 untreated, recent onset RA subjects, 20 HLA, age, and sex matched healthy controls and 18 other subjects with inflammatory arthritis. PBMC proliferative responses to a second E coli dnaJ peptide (with the shared epitope at the N-terminus) and two peptides from type II collagen with high affinity for DR4(0401) were tested in a further 16 recent onset RA and 17 control subjects. RESULTS—PBMC proliferation and whole blood interferon γ or interleukin 10 production in response to the shared epitope containing and control peptides were not different between the disease and control groups. On the other hand, compared with controls, RA subjects had significantly higher proliferation to a collagen II (aa 1307-1319) peptide, but significantly lower proliferation and interferon γ production to tetanus toxoid. CONCLUSION—Recent onset RA subjects had no demonstrable increase in peripheral blood T cell reactivity to shared epitope containing peptides. However, a proportion had increased T cell reactivity to a peptide of similar length from a candidate RA autoantigen, collagen type II. Their impaired responses to tetanus are in keeping with evidence for general T cell hyporesponsiveness in RA.
Full Text
The Full Text of this article is available as a PDF (158.9 KB).
Figure 1 .
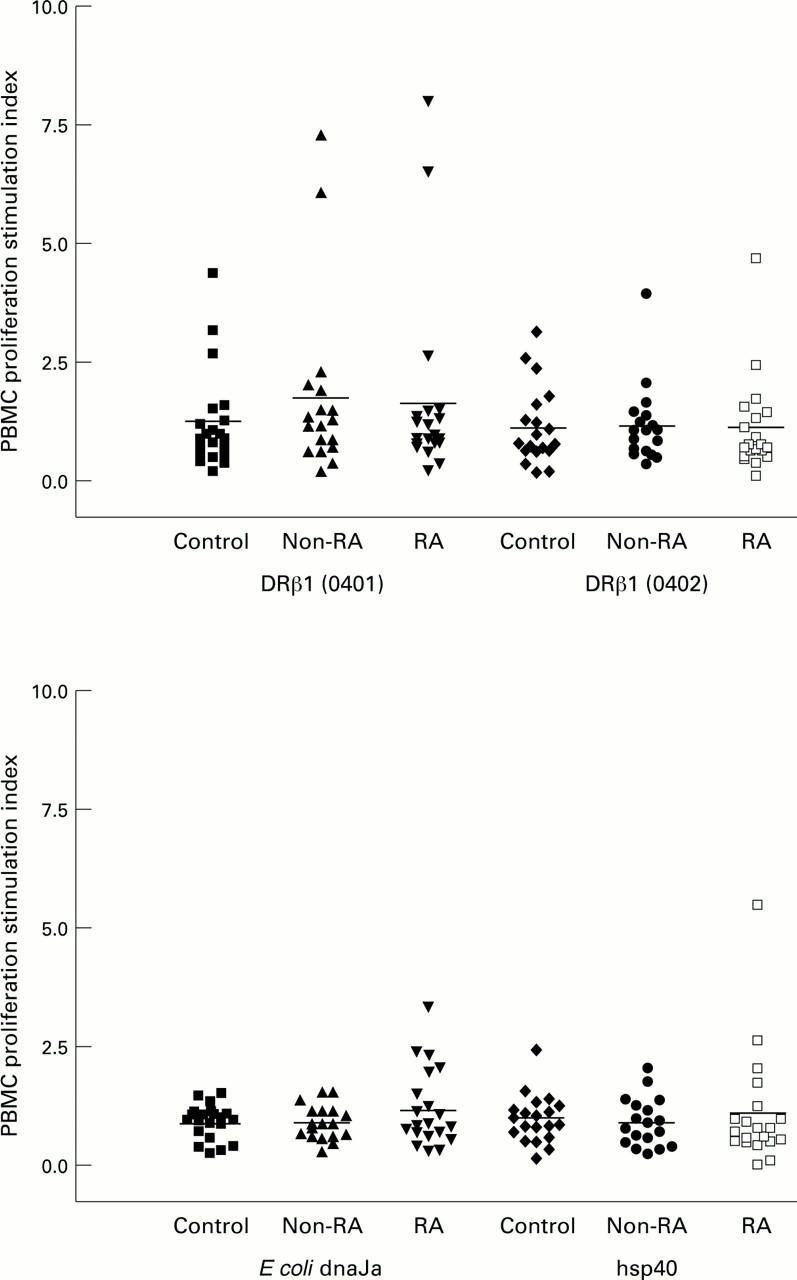
Stimulation indices for proliferation of PBMCs from RA, non-RA disease control, and healthy control (study 1) subjects after incubation with 50 µg/ml of DRβ1(0401), DRβ1(0402), E coli dnaJa (aa 56-70) or hsp40 peptides.
Figure 2 .
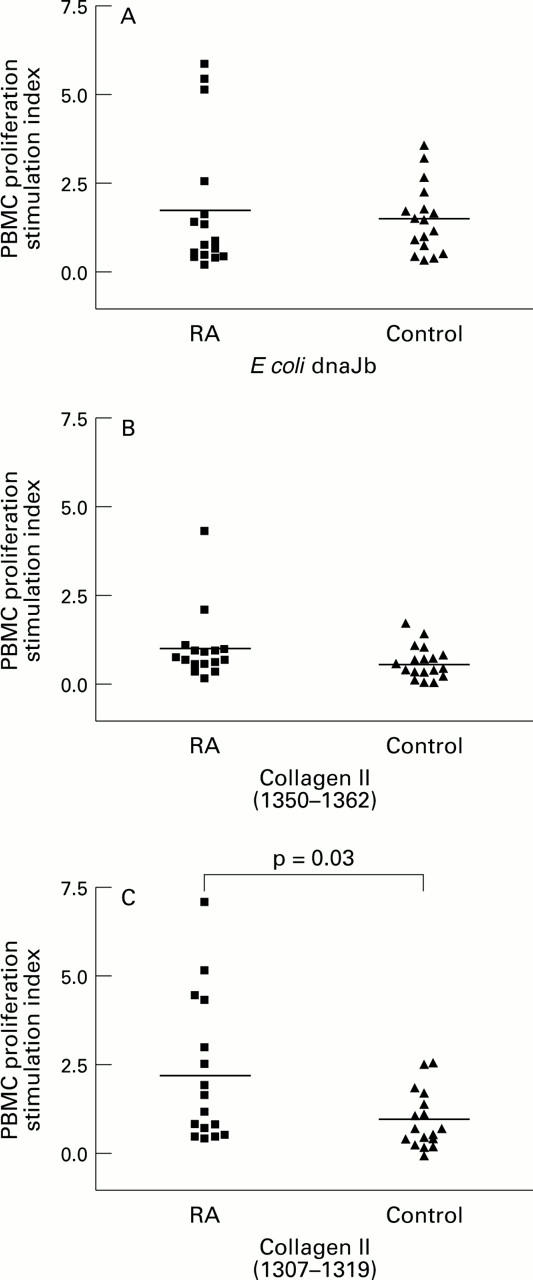
Stimulation indices for proliferation of PBMCs from a second group of RA and control (study 2) subjects after incubation with (A) E coli dnaJb (aa 61-75) peptide;(B) collagen type II (aa 1350-1362) peptide; (C) collagen type II (aa 1307-1319) peptide.
Figure 3 .
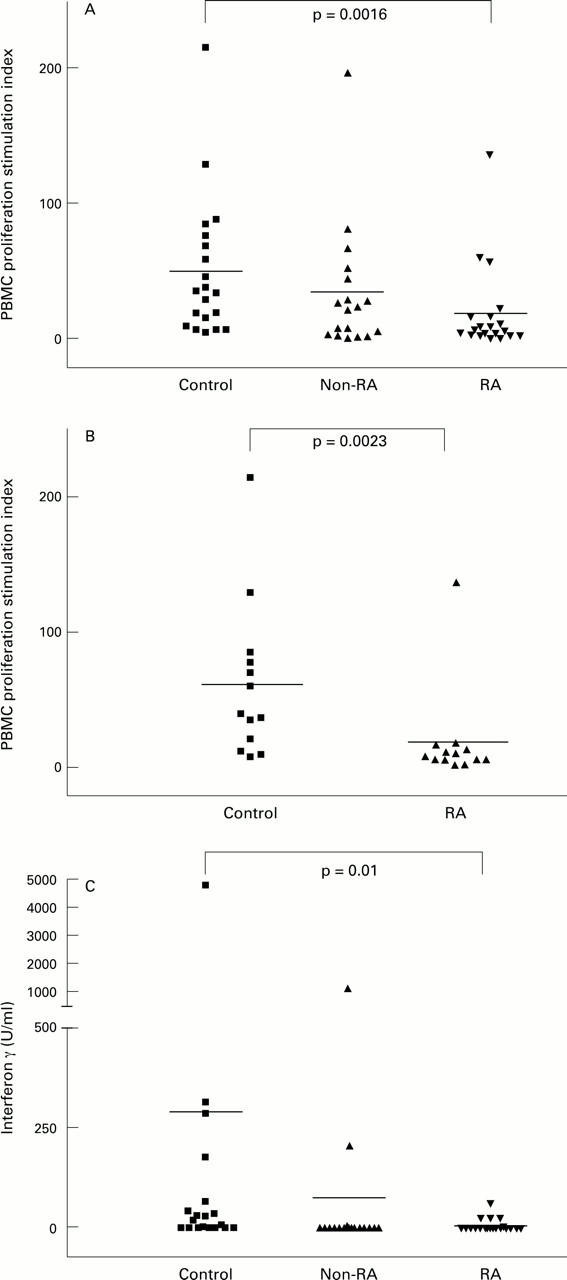
(A) Stimulation indices for proliferation of PBMCs from RA, non-RA disease control, and healthy control (study 1) subjects after incubation with 2 Lfu/ml of tetanus toxoid; (B) stimulation indices for proliferation of PBMCs from DR4 expressing study 1 subjects after incubation with 2 Lfu/ml of tetanus toxoid; (C) interferon γ production in whole blood from study 1 subjects after incubation with 1.3 Lfu/ml of tetanus toxoid.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani S., Keystone E. C., Nelson J. L., Ollier W. E., La Cava A., Montemayor A. C., Weber D. A., Montecucco C., Martini A., Carson D. A. Positive selection in autoimmunity: abnormal immune responses to a bacterial dnaJ antigenic determinant in patients with early rheumatoid arthritis. Nat Med. 1995 May;1(5):448–452. doi: 10.1038/nm0595-448. [DOI] [PubMed] [Google Scholar]
- Albani S., Roudier J. Molecular basis for the association between HLA DR4 and rheumatoid arthritis. From the shared epitope hypothesis to a peptidic model of rheumatoid arthritis. Clin Biochem. 1992 Jun;25(3):209–212. doi: 10.1016/0009-9120(92)90328-p. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Cohen S. B., Katsikis P. D., Chu C. Q., Thomssen H., Webb L. M., Maini R. N., Londei M., Feldmann M. High level of interleukin-10 production by the activated T cell population within the rheumatoid synovial membrane. Arthritis Rheum. 1995 Jul;38(7):946–952. doi: 10.1002/art.1780380710. [DOI] [PubMed] [Google Scholar]
- Cope A. P., Londei M., Chu N. R., Cohen S. B., Elliott M. J., Brennan F. M., Maini R. N., Feldmann M. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994 Aug;94(2):749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. K., DelGiudice-Asch G., Zehetbauer J. B., Lawson J. L., Brot N., Weissbach H., Elkon K. B. Autoantigen-specific T cell proliferation induced by the ribosomal P2 protein in patients with systemic lupus erythematosus. J Clin Invest. 1994 Jul;94(1):345–352. doi: 10.1172/JCI117328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Panayi G. S., Nouri A. M. Interleukin-2 reverses deficient cell-mediated immune responses in rheumatoid arthritis. Clin Exp Immunol. 1984 Jul;57(1):123–129. [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J. Functional and biosynthetic changes in endothelial cells of vessels in chronically inflamed tissues: evidence for endothelial control of lymphocyte entry into diseased tissues. J Pathol. 1988 Jul;155(3):225–230. doi: 10.1002/path.1711550308. [DOI] [PubMed] [Google Scholar]
- Freemont A. J. Molecules controlling lymphocyte-endothelial interactions in lymph nodes are produced in vessels of inflamed synovium. Ann Rheum Dis. 1987 Dec;46(12):924–928. doi: 10.1136/ard.46.12.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Hammer J., Gallazzi F., Bono E., Karr R. W., Guenot J., Valsasnini P., Nagy Z. A., Sinigaglia F. Peptide binding specificity of HLA-DR4 molecules: correlation with rheumatoid arthritis association. J Exp Med. 1995 May 1;181(5):1847–1855. doi: 10.1084/jem.181.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraoui B., Wilder R. L., Malone D. G., Allen J. B., Katona I. M., Wahl S. M. Immune function in severe, active rheumatoid arthritis: a relationship between peripheral blood mononuclear cell proliferation to soluble antigens and mononuclear cell subset profiles. J Immunol. 1984 Aug;133(2):697–701. [PubMed] [Google Scholar]
- Harris P. E. Self-peptides bound to HLA molecules. Crit Rev Immunol. 1994;14(1):61–87. [PubMed] [Google Scholar]
- Keystone E., Poplonski L., Snow K. M., Shore A., Schiavone A., Narendran A., Harth M. Reconstitution of impaired autologous mixed lymphocyte reactivity in rheumatoid arthritis. Autoimmunity. 1991;8(3):199–207. doi: 10.3109/08916939108997107. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Davis L. S., Cush J. J., Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Malaise M. G., Franchimont P. Defective in vitro gamma-interferon production in rheumatoid arthritis. Arthritis Rheum. 1987 Feb;30(2):230–231. doi: 10.1002/art.1780300217. [DOI] [PubMed] [Google Scholar]
- Malone D. G., Wahl S. M., Tsokos M., Cattell H., Decker J. L., Wilder R. L. Immune function in severe, active rheumatoid arthritis. A relationship between peripheral blood mononuclear cell proliferation to soluble antigens and synovial tissue immunohistologic characteristics. J Clin Invest. 1984 Oct;74(4):1173–1185. doi: 10.1172/JCI111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Paimela L., Johansson-Stephansson E. A., Koskimies S., Leirisalo-Repo M. Depressed cutaneous cell-mediated immunity in early rheumatoid arthritis. Clin Exp Rheumatol. 1990 Sep-Oct;8(5):433–437. [PubMed] [Google Scholar]
- Petrovsky N., Harrison L. C. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995 Oct 12;186(1):37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- Pope R. M., Kniker W. T., Talal N., Dauphinee M. Delayed type hypersensitivity in patients with rheumatoid arthritis. J Rheumatol. 1993 Jan;20(1):17–20. [PubMed] [Google Scholar]
- Reuter A., Bernier J., Vrindts-Gevaert Y., Meuleman-Gathy R., Malaise M., Fiers W., Franchimont P. Production of interferon gamma by peripheral blood mononuclear cells from normal subjects and from patients with rheumatoid arthritis. Clin Exp Rheumatol. 1988 Oct-Dec;6(4):347–354. [PubMed] [Google Scholar]
- Rudensky A. Y. Endogenous peptides associated with MHC class II and selection of CD4 T cells. Semin Immunol. 1995 Dec;7(6):399–409. doi: 10.1006/smim.1995.0044. [DOI] [PubMed] [Google Scholar]
- Salmi M., Andrew D. P., Butcher E. C., Jalkanen S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J Exp Med. 1995 Jan 1;181(1):137–149. doi: 10.1084/jem.181.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvat S., Auger I., Rochelle L., Begovich A., Geburher L., Sette A., Roudier J. Tolerance to a self-peptide from the third hypervariable region of HLA DRB1*0401 in rheumatoid arthritis patients and normal subjects. J Immunol. 1994 Dec 1;153(11):5321–5329. [PubMed] [Google Scholar]
- Seitz M., Napierski I., Kirchner H. Depressed PPD and tetanus toxoid presentation by monocytes to T lymphocytes in patients with rheumatoid arthritis: restoration by interferon gamma. Rheumatol Int. 1988;8(5):189–196. doi: 10.1007/BF00269194. [DOI] [PubMed] [Google Scholar]
- Sharma S. K. Endotoxin detection and elimination in biotechnology. Biotechnol Appl Biochem. 1986 Feb;8(1):5–22. [PubMed] [Google Scholar]
- Silman A. J., MacGregor A. J., Thomson W., Holligan S., Carthy D., Farhan A., Ollier W. E. Twin concordance rates for rheumatoid arthritis: results from a nationwide study. Br J Rheumatol. 1993 Oct;32(10):903–907. doi: 10.1093/rheumatology/32.10.903. [DOI] [PubMed] [Google Scholar]
- Stolzenburg T., Binz H., Fontana A., Felder M., Wagenhaeuser F. J. Impaired mitogen-induced interferon-gamma production in rheumatoid arthritis and related diseases. Scand J Immunol. 1988 Jan;27(1):73–81. doi: 10.1111/j.1365-3083.1988.tb02324.x. [DOI] [PubMed] [Google Scholar]
- Tsokos G. C., Boumpas D. T., Smith P. L., Djeu J. Y., Balow J. E., Rook A. H. Deficient gamma-interferon production in patients with systemic lupus erythematosus. Arthritis Rheum. 1986 Oct;29(10):1210–1215. doi: 10.1002/art.1780291005. [DOI] [PubMed] [Google Scholar]
- Verwilghen J., Vertessen S., Stevens E. A., Dequeker J., Ceuppens J. L. Depressed T-cell reactivity to recall antigens in rheumatoid arthritis. J Clin Immunol. 1990 Mar;10(2):90–98. doi: 10.1007/BF00918190. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wilder R. L., Katona I. M., Wahl L. M., Allen J. B., Scher I., Decker J. L. Leukapheresis in rheumatoid arthritis. Association of clinical improvement with reversal of anergy. Arthritis Rheum. 1983 Sep;26(9):1076–1084. doi: 10.1002/art.1780260904. [DOI] [PubMed] [Google Scholar]
- Zangerle P. F., De Groote D., Lopez M., Meuleman R. J., Vrindts Y., Fauchet F., Dehart I., Jadoul M., Radoux D., Franchimont P. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992 Nov;4(6):568–575. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]


