Full Text
The Full Text of this article is available as a PDF (179.4 KB).
Figure 1 .
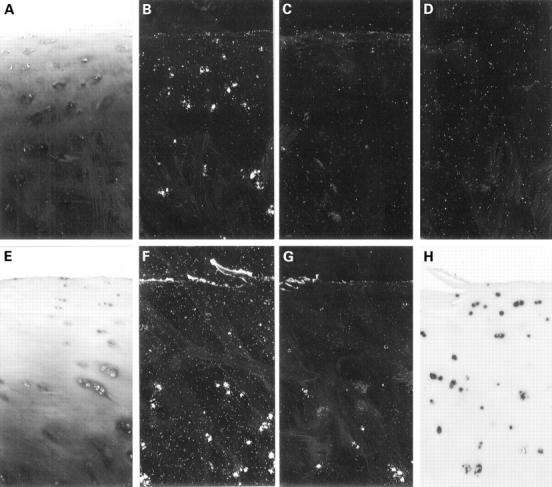
In situ hybridisation analysis of normal (A-D) and osteoarthritic (E-H) cartilage specimens using RNA probes specific for the major extracellular matrix proteins of articular cartilage, aggrecan core protein (B,F), and collagen type II (C,G). Chondrocytes of normal articular cartilage from all specimens showed no significant expression of collagen type II mRNA (C), but significant levels of aggrecan core protein mRNA in most cases (B). In contrast, analysis of osteoarthritic cartilage showed a strong activation of the expression of collagen type II mRNA in the middle and upper deep zone chondrocytes in all samples (G). Aggrecan mRNA expression was, in comparison, much less increased in these cells (F) compared with the normal specimens. Thus, mRNA analysis showed osteoarthritic chondrocytes to be hyperactive in the middle and deeper zones of cartilage compared with normal specimens. Most importantly however, in the upper zone chondrocytes in most of the osteoarthritic specimens, no expression of either protein was observed (F,G). Coincidentally with this area, loss of proteoglycan staining was seen (E). The upper inactive chondrocytes were not necrotic as shown by strong signals for 18S rRNA (H). (A,E: toluidine blue; B-D,F,G: dark fields; A-D: femoral head, 68 years, Mankin's grade 1, female; E-H: femoral head, 69 years, Mankin's grade 4, female; exposure time: B-D,F: three weeks; G: four days; A-H:original magnification × 140; the chosen exposure times were optimal. Longer exposures, did not increase the number of positive cells nor improve the signal to noise ratio). Methods (figs 1 and 2): 11 normal specimens from necopsies and amputations for cancer (age range 45 to 79 years) and 32 osteoarthritic cartilage slices (hip and knee replacement operations for late stage osteoarthritis; age range 52-78 years) were fixed with 4% paraformaldehyde, decalcified, and embedded in paraffin wax. Toluidine blue and safranin O stainings were performed to estimate the content of proteoglycans.41 The samples were classified and graded according to Mankin et al.6 Specific 35S-labelled RNA probes for human collagen chains α1(I), α1(II), α1(III), α1(X), and aggrecan core protein were prepared and in situ hybridisation performed as described elsewhere.21 25 The riboprobe for 18S rRNA (H) was digoxigenin labelled and detected according to the manufacturer's protocol (Boehringer, Mannheim, Germany). Control experiments: the specificity of the cDNA probes was ascertained by computerised homology search and in situ hybridisation experiments in the fetal growth plate.21 Sense transcripts were used as non-specific negative controls and did not give signals above background (D). Immunohistochemistry: deparaffinised sections were pretreated with testicular hyaluronidase and pronase. Primary antibodies were incubated for one hour and visualised using alkaline-phosphatase-labelled secondary antibodies. Nuclei were counterstained with haematoxylin. Polyclonal rabbit antisera against human type I collagen and monoclonal antibodies against type X collagen were prepared as described elsewhere.27 42 Monoclonal antibodies against type II collagen (CIID3) were kindly provided by Dr R Holmdahl (Uppsala, Sweden43). Polyclonal antibodies against type III procollagen were kindly provided by Dr Günzler (Frankfurt, Germany).
Figure 2 .
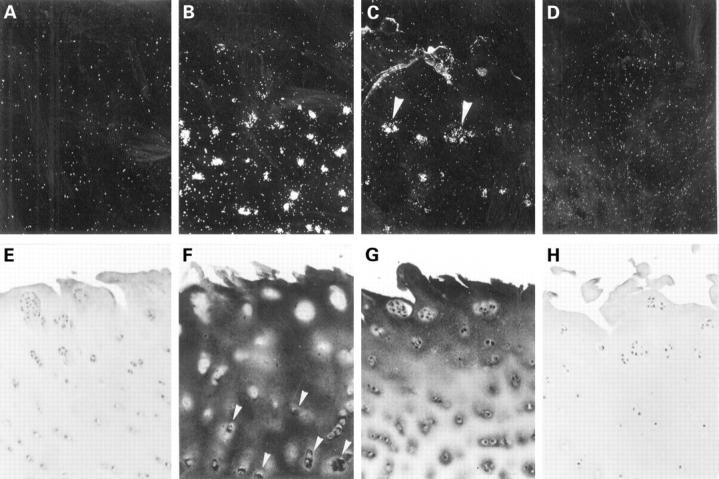
Phenotyping of osteoarthritic chondrocytes: in situ hybridisation analysis of chondrocytes revealed in all osteoarthritic specimens collagen type II mRNA expression in the middle zones (B), where intracellular staining for type II collagen was also found immunohistochemically (F; arrow heads). In chondrocytes of the upper zone the expression of collagen type II ceased. Interestingly, in the upper middle zone an onset of type III collagen mRNA expression was observed in most samples (C). This was confirmed by immunohistochemical staining for collagen type III (G). Neither collagen type I (A,E) nor collagen type X (D,H) expression or deposition, as marker collagens of dedifferentiated and hypertrophic chondrocytes, respectively, was observed in the upper and middle zones of osteoarthritic cartilage samples. Except for some weak signals for collagen type II, normal cartilage samples did not show expression of any of the collagens (not shown). (A-D: dark fields; femoral head, 69 years, female; Mankin's grade 5; exposure time: A,C,D: three weeks; B: four days; original magnification × 100).
Figure 3 .
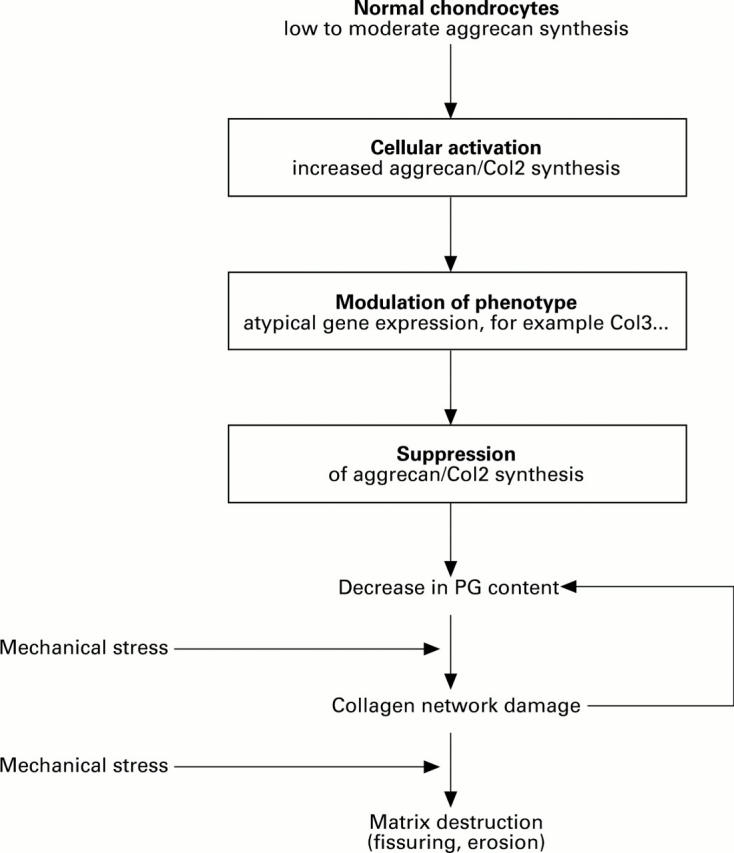
Schematic representation of the three steps of cellular events in osteoarthritic cartilage degeneration as proposed by the hypothesis: (1) cellular activation of chondrocytes, (2) modulation of the cellular phenotype, and (3) suppression of anabolic activity. This leads to a quantitative loss of aggrecan molecules from the extracellular matrix and to a collagen network damage, which again promotes further loss of proteoglycans. Finally, fissuring and complete destruction of the cartilage matrix occurs.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aigner T., Bertling W., Stöss H., Weseloh G., von der Mark K. Independent expression of fibril-forming collagens I, II, and III in chondrocytes of human osteoarthritic cartilage. J Clin Invest. 1993 Mar;91(3):829–837. doi: 10.1172/JCI116303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner T., Dietz U., Stöss H., von der Mark K. Differential expression of collagen types I, II, III, and X in human osteophytes. Lab Invest. 1995 Aug;73(2):236–243. [PubMed] [Google Scholar]
- Aigner T., Stöss H., Weseloh G., Zeiler G., von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(6):337–345. doi: 10.1007/BF02899701. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978 Dec;15(4):1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bonaventure J., Kadhom N., Cohen-Solal L., Ng K. H., Bourguignon J., Lasselin C., Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994 May;212(1):97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Descalzi Cancedda F., Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. doi: 10.1016/s0074-7696(08)62109-9. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré S., Pelletier J. P., DiBattista J. A., Tardif G., Brazeau P., Martel-Pelletier J. Human osteoarthritic chondrocytes possess an increased number of insulin-like growth factor 1 binding sites but are unresponsive to its stimulation. Possible role of IGF-1-binding proteins. Arthritis Rheum. 1994 Feb;37(2):253–263. doi: 10.1002/art.1780370215. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., McDevitt C. A., Billingham M. E., Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980 Jun 15;188(3):823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechtenmacher J., Huch K., Thonar E. J., Mollenhauer J. A., Davies S. R., Schmid T. M., Puhl W., Sampath T. K., Aydelotte M. B., Kuettner K. E. Recombinant human osteogenic protein 1 is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum. 1996 Nov;39(11):1896–1904. doi: 10.1002/art.1780391117. [DOI] [PubMed] [Google Scholar]
- Girkontaite I., Frischholz S., Lammi P., Wagner K., Swoboda B., Aigner T., Von der Mark K. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996 Sep;15(4):231–238. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- Grushko G., Schneiderman R., Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19(2-4):149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- Harrison E. T., Jr, Luyten F. P., Reddi A. H. Osteogenin promotes reexpression of cartilage phenotype by dedifferentiated articular chondrocytes in serum-free medium. Exp Cell Res. 1991 Feb;192(2):340–345. doi: 10.1016/0014-4827(91)90050-5. [DOI] [PubMed] [Google Scholar]
- Kato Y., Gospodarowicz D. Growth requirements of low-density rabbit costal chondrocyte cultures maintained in serum-free medium. J Cell Physiol. 1984 Sep;120(3):354–363. doi: 10.1002/jcp.1041200314. [DOI] [PubMed] [Google Scholar]
- Lafeber F. P., van der Kraan P. M., van Roy H. L., Vitters E. L., Huber-Bruning O., van den Berg W. B., Bijlsma J. W. Local changes in proteoglycan synthesis during culture are different for normal and osteoarthritic cartilage. Am J Pathol. 1992 Jun;140(6):1421–1429. [PMC free article] [PubMed] [Google Scholar]
- Lippiello L., Hall D., Mankin H. J. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977 Apr;59(4):593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. B., Ghosh P., Bellenger C. R. Topographic variation in biglycan and decorin synthesis by articular cartilage in the early stages of osteoarthritis: an experimental study in sheep. J Orthop Res. 1996 May;14(3):433–444. doi: 10.1002/jor.1100140314. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mankin H. J., Johnson M. E., Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar-containing macromolecules. J Bone Joint Surg Am. 1981 Jan;63(1):131–139. [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Matyas J. R., Adams M. E., Huang D., Sandell L. J. Discoordinate gene expression of aggrecan and type II collagen in experimental osteoarthritis. Arthritis Rheum. 1995 Mar;38(3):420–425. doi: 10.1002/art.1780380320. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrovic D., Gruson M., Demignon J., Mercier P., Aprile F., De Seze S. Metabolism of human femoral head cartilage in osteoarthrosis and subcapital fracture. Ann Rheum Dis. 1981 Feb;40(1):18–26. doi: 10.1136/ard.40.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Rayan V., Hardingham T. The recovery of articular cartilage in explant culture from interleukin-1 alpha: effects on proteoglycan synthesis and degradation. Matrix Biol. 1994 Apr;14(3):263–271. doi: 10.1016/0945-053x(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Reichenberger E., Aigner T., von der Mark K., Stöss H., Bertling W. In situ hybridization studies on the expression of type X collagen in fetal human cartilage. Dev Biol. 1991 Dec;148(2):562–572. doi: 10.1016/0012-1606(91)90274-7. [DOI] [PubMed] [Google Scholar]
- Rizkalla G., Reiner A., Bogoch E., Poole A. R. Studies of the articular cartilage proteoglycan aggrecan in health and osteoarthritis. Evidence for molecular heterogeneity and extensive molecular changes in disease. J Clin Invest. 1992 Dec;90(6):2268–2277. doi: 10.1172/JCI116113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Roughley P. J., Lee E. R. Cartilage proteoglycans: structure and potential functions. Microsc Res Tech. 1994 Aug 1;28(5):385–397. doi: 10.1002/jemt.1070280505. [DOI] [PubMed] [Google Scholar]
- Sailor L. Z., Hewick R. M., Morris E. A. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996 Nov;14(6):937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Schmid T. M., Popp R. G., Linsenmayer T. F. Hypertrophic cartilage matrix. Type X collagen, supramolecular assembly, and calcification. Ann N Y Acad Sci. 1990;580:64–73. doi: 10.1111/j.1749-6632.1990.tb17918.x. [DOI] [PubMed] [Google Scholar]
- Sweet M. B., Thonar E. J., Immelman A. R., Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis. 1977 Oct;36(5):387–398. doi: 10.1136/ard.36.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Vornehm S. I., Dudhia J., Von der Mark K., Aigner T. Expression of collagen types IX and XI and other major cartilage matrix components by human fetal chondrocytes in vivo. Matrix Biol. 1996 Jul;15(2):91–98. doi: 10.1016/s0945-053x(96)90150-x. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Gauss V., von der Mark H., Müller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977 Jun 9;267(5611):531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- von der Mark K., von der Mark H., Gay S. Study of differential collagen synthesis during development of the chick embryo by immunofluroescence. II. Localization of type I and type II collagen during long bone development. Dev Biol. 1976 Oct 15;53(2):153–170. doi: 10.1016/0012-1606(76)90220-7. [DOI] [PubMed] [Google Scholar]


