Abstract
OBJECTIVES—To determine concentrations of chondroitin sulphate (CS) and keratan sulphate (KS) epitopes, glycosaminoglycans (GAGs) and hyaluronan (HA) in knee synovial fluid (SF) from normal subjects and patients with osteoarthritis (OA) or rheumatoid arthritis (RA), to test whether these variables may be used as markers of the OA process. METHODS—OA was subdivided into large joint OA (LJOA), nodal generalised OA (NGOA), and OA with calcium pyrophosphate crystal deposition (CPA). Clinical assessment of inflammation (0-6) was undertaken on OA and RA knees. Knee SF was examined by enzyme linked immunosorbent assay for: CS epitopes, using monoclonal antibodies 3-B-3 and 7-D-4; KS epitope using monoclonal antibody 5-D-4; and HA, using biotinylated HA binding region of cartilage proteoglycan. Total sulphated GAGs were measured by dye binding with 1:9 dimethylmethylene blue. RESULTS—Increased SF 3-B-3 concentrations and 3-B-3/GAG ratio were found in OA, compared with RA or normal knees, with higher 3-B-3 and 3-B-3/GAG in LJOA and NGOA than in CPA. SF 7-D-4 and 7-D-4/GAG were reduced in RA, compared with normal and OA; SF 5-D-4 was reduced in OA compared with normal. GAG and HA concentrations were decreased in both OA and RA. No correlations with radiographic scores were observed, but SF 7-D-4 was lower in `inflamed' compared with `non-inflamed' RA and OA knees. In patients with bilateral samples there were strong correlations between right and left knees for all SF variables. CONCLUSIONS—Changed concentrations of SF CS and KS can be detected in OA with a profile that differs from that seen in RA. Clinical subgrouping and local joint inflammation may influence these measures, supporting different pathogenesis within OA subgroups and requirement for careful patient characterisation in SF studies.
Full Text
The Full Text of this article is available as a PDF (201.8 KB).
Figure 1 .
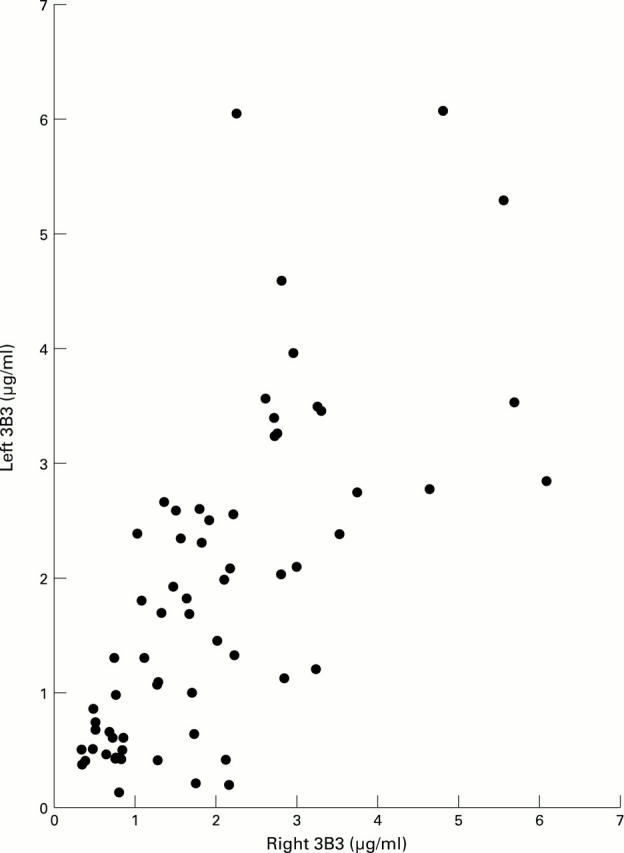
Correlation between measurement of 3-B-3 in left and right knees of individual patients. When tested by Spearman's rank correlation, r=0.7324, p<0.001.
Figure 2 .
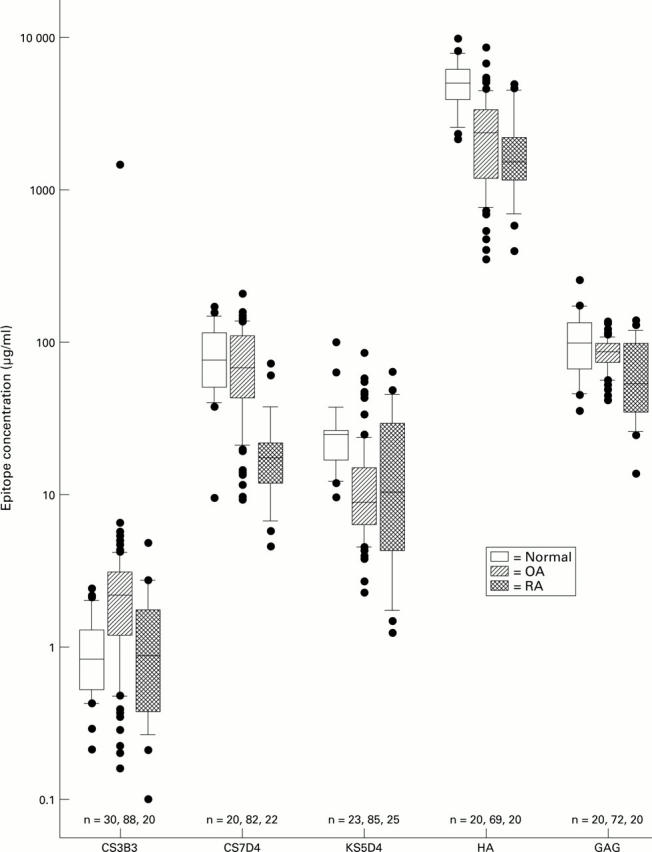
Comparison between RA, OA, and normal knees for all epitopes measured. The central solid line represents the median, with the box representing the middle 50% of the data. The error bar cap lines mark the 10th and 90th percentiles. Dots represent individual data points outside the 10th and 90th percentiles.
Figure 3 .
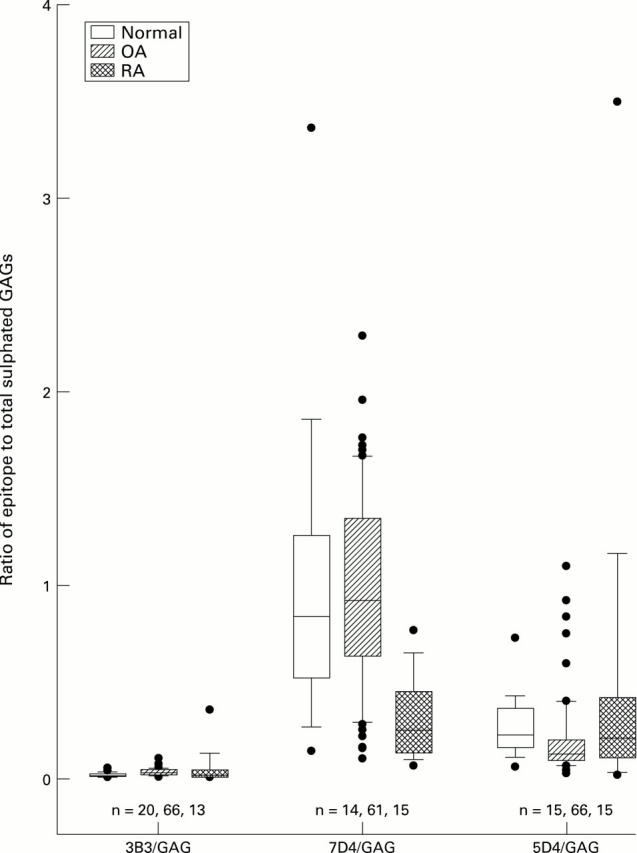
Comparison between RA, OA, and normal knees for ratio of chondroitin sulphate and keratan sulphate epitopes to total sulphated glycosaminoglycans. The central solid line represents the median, with the box representing the middle 50% of the data. The error bar cap lines mark the 10th and 90th percentiles. Dots represent individual data points outside the 10th and 90th percentiles.
Figure 4 .

Comparison between the OA subclasses NGOA, LJOA, and CPA for all epitopes measured. The central solid line represents the median, with the box representing the middle 50% of the data. The error bar cap lines mark the 10th and 90th percentiles. Dots represent individual data points outside the 10th and 90th percentiles.
Figure 5 .
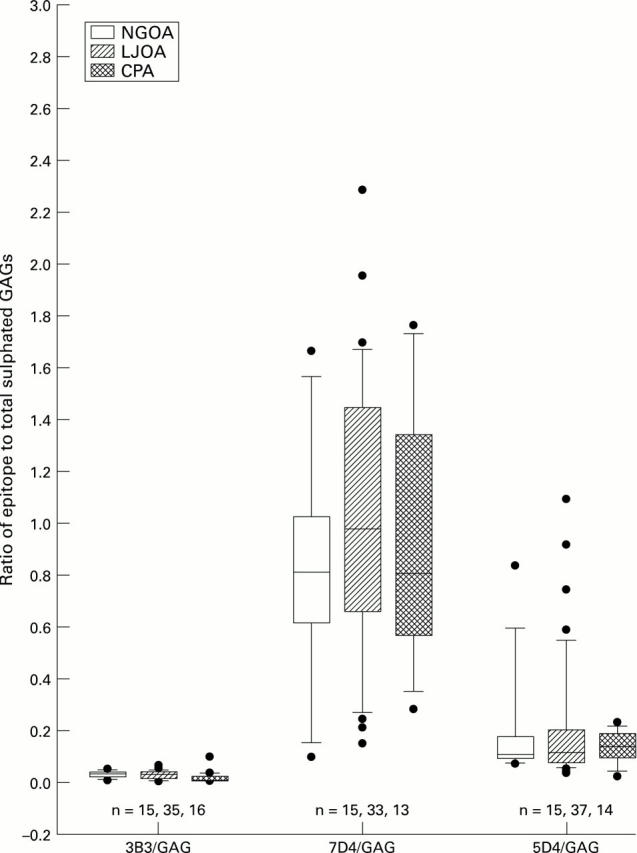
Comparison between the OA subclasses NGOA, LJOA, and CPA for ratio of chondroitin sulphate and keratan sulphate epitopes to total sulphated glycosaminoglycans. The central solid line represents the median, with the box representing the middle 50% of the data. The error bar cap lines mark the 10th and 90th percentiles. Dots represent individual data points outside the 10th and 90th percentiles.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balazs E. A., Watson D., Duff I. F., Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967 Aug;10(4):357–376. doi: 10.1002/art.1780100407. [DOI] [PubMed] [Google Scholar]
- Belcher C., Fawthrop F., Bunning R., Doherty M. Plasminogen activators and their inhibitors in synovial fluids from normal, osteoarthritis, and rheumatoid arthritis knees. Ann Rheum Dis. 1996 Apr;55(4):230–236. doi: 10.1136/ard.55.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensouyad A., Hollander A. P., Dularay B., Bedwell A. E., Cooper R. A., Hutton C. W., Dieppe P. A., Elson C. J. Concentrations of glycosaminoglycans in synovial fluids and their relation with immunological and inflammatory mediators in rheumatoid arthritis. Ann Rheum Dis. 1990 May;49(5):301–307. doi: 10.1136/ard.49.5.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J. A., Schnitzer T. J., Andersson G. B., Lenz M. E., Jeffery R., McNeill T. W., Thonar E. J. The effect of chemonucleolysis on serum keratan sulfate levels in humans. Arthritis Rheum. 1989 Jan;32(1):100–104. doi: 10.1002/anr.1780320118. [DOI] [PubMed] [Google Scholar]
- Campion G. V., McCrae F., Schnitzer T. J., Lenz M. E., Dieppe P. A., Thonar E. J. Levels of keratan sulfate in the serum and synovial fluid of patients with osteoarthritis of the knee. Arthritis Rheum. 1991 Oct;34(10):1254–1259. doi: 10.1002/art.1780341008. [DOI] [PubMed] [Google Scholar]
- Caterson B., Mahmoodian F., Sorrell J. M., Hardingham T. E., Bayliss M. T., Carney S. L., Ratcliffe A., Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990 Nov;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Cs-Szabó G., Roughley P. J., Plaas A. H., Glant T. T. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995 May;38(5):660–668. doi: 10.1002/art.1780380514. [DOI] [PubMed] [Google Scholar]
- Dahl L. B., Dahl I. M., Engström-Laurent A., Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985 Dec;44(12):817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M., Belcher C., Regan M., Jones A., Ledingham J. Association between synovial fluid levels of inorganic pyrophosphate and short term radiographic outcome of knee osteoarthritis. Ann Rheum Dis. 1996 Jul;55(7):432–436. doi: 10.1136/ard.55.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M., Richards N., Hornby J., Powell R. Relation between synovial fluid C3 degradation products and local joint inflammation in rheumatoid arthritis, osteoarthritis, and crystal associated arthropathy. Ann Rheum Dis. 1988 Mar;47(3):190–197. doi: 10.1136/ard.47.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fawthrop F., Yaqub R., Belcher C., Bayliss M., Ledingham J., Doherty M. Chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in progressive versus non-progressive osteoarthritis. Ann Rheum Dis. 1997 Feb;56(2):119–122. doi: 10.1136/ard.56.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Hey N. J., Carney S. L., Hardingham T. E. An ELISA plate-based assay for hyaluronan using biotinylated proteoglycan G1 domain (HA-binding region). Matrix. 1990 Oct;10(5):306–313. doi: 10.1016/s0934-8832(11)80186-1. [DOI] [PubMed] [Google Scholar]
- Hamilton E., Pattrick M., Doherty M. Inorganic pyrophosphate, nucleoside triphosphate pyrophosphatase, and cartilage fragments in normal human synovial fluid. Br J Rheumatol. 1991 Aug;30(4):260–264. doi: 10.1093/rheumatology/30.4.260. [DOI] [PubMed] [Google Scholar]
- Hardingham T., Bayliss M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin Arthritis Rheum. 1990 Dec;20(3 Suppl 1):12–33. doi: 10.1016/0049-0172(90)90044-g. [DOI] [PubMed] [Google Scholar]
- Hazell P. K., Dent C., Fairclough J. A., Bayliss M. T., Hardingham T. E. Changes in glycosaminoglycan epitope levels in knee joint fluid following injury. Arthritis Rheum. 1995 Jul;38(7):953–959. doi: 10.1002/art.1780380711. [DOI] [PubMed] [Google Scholar]
- Jones A., Hopkinson N., Pattrick M., Berman P., Doherty M. Evaluation of a method for clinically assessing osteoarthritis of the knee. Ann Rheum Dis. 1992 Feb;51(2):243–245. doi: 10.1136/ard.51.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledingham J., Regan M., Jones A., Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Ann Rheum Dis. 1993 Jul;52(7):520–526. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Mehraban F., Finegan C. K., Moskowitz R. W. Serum keratan sulfate. Quantitative and qualitative comparisons in inflammatory versus noninflammatory arthritides. Arthritis Rheum. 1991 Apr;34(4):383–392. doi: 10.1002/art.1780340403. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Doherty M., Maini R. N., Hardingham T. E. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Ann Rheum Dis. 1988 Oct;47(10):826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Shurety W., Caterson B. The quantitation of a native chondroitin sulfate epitope in synovial fluid lavages and articular cartilage from canine experimental osteoarthritis and disuse atrophy. Arthritis Rheum. 1993 Apr;36(4):543–551. doi: 10.1002/art.1780360416. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Synovial fluid analysis of two groups of proteoglycan epitopes distinguishes early and late cartilage lesions. Arthritis Rheum. 1992 Apr;35(4):385–390. doi: 10.1002/art.1780350404. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A., Pettersson H. Difference in cartilage proteoglycan level in synovial fluid in early rheumatoid arthritis and reactive arthritis. Lancet. 1985 Jul 20;2(8447):127–128. doi: 10.1016/s0140-6736(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Sharif M., George E., Shepstone L., Knudson W., Thonar E. J., Cushnaghan J., Dieppe P. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum. 1995 Jun;38(6):760–767. doi: 10.1002/art.1780380608. [DOI] [PubMed] [Google Scholar]
- Sharif M., Osborne D. J., Meadows K., Woodhouse S. M., Colvin E. M., Shepstone L., Dieppe P. A. The relevance of chondroitin and keratan sulphate markers in normal and arthritic synovial fluid. Br J Rheumatol. 1996 Oct;35(10):951–957. doi: 10.1093/rheumatology/35.10.951. [DOI] [PubMed] [Google Scholar]
- Silverman B., Cawston T. E., Page Thomas D. P., Dingle J. T., Hazleman B. L. The sulphated glycosaminoglycan levels in synovial fluid aspirates in patients with acute and chronic joint disease. Br J Rheumatol. 1990 Oct;29(5):340–344. doi: 10.1093/rheumatology/29.5.340. [DOI] [PubMed] [Google Scholar]
- Slater R. R., Jr, Bayliss M. T., Lachiewicz P. F., Visco D. M., Caterson B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995 May;38(5):655–659. doi: 10.1002/art.1780380513. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Woodward L., Hall G. M., Hammond A., Williams A., Butler M. G., James I. T., Hart D. J., Thompson P. W., Scott D. L. Keratan sulphate in rheumatoid arthritis, osteoarthritis, and inflammatory diseases. Ann Rheum Dis. 1992 Oct;51(10):1134–1137. doi: 10.1136/ard.51.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Lenz M. E., Klintworth G. K., Caterson B., Pachman L. M., Glickman P., Katz R., Huff J., Kuettner K. E. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 1985 Dec;28(12):1367–1376. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]
- Visco D. M., Johnstone B., Hill M. A., Jolly G. A., Caterson B. Immunohistochemical analysis of 3-B-(-) and 7-D-4 epitope expression in canine osteoarthritis. Arthritis Rheum. 1993 Dec;36(12):1718–1725. doi: 10.1002/art.1780361211. [DOI] [PubMed] [Google Scholar]
- Williams J. M., Downey C., Thonar E. J. Increase in levels of serum keratan sulfate following cartilage proteoglycan degradation in the rabbit knee joint. Arthritis Rheum. 1988 Apr;31(4):557–560. doi: 10.1002/art.1780310415. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Glynn R. J., Felson D. T. Musculoskeletal disease research: should we analyze the joint or the person? J Rheumatol. 1996 Jul;23(7):1130–1134. [PubMed] [Google Scholar]


