Abstract
OBJECTIVE—To discover if α smooth muscle actin expression and myofibroblastic differentiation are induced in synovial fibroblasts by cytokines found in the inflamed RA joint. METHODS—Immunofluorescent microscopy and western blotting were used to examine different cultures of human synovial fibroblasts for expression of α actin in the presence of the cytokines transforming growth factor β (TGFβ1), interleukin 1α (IL1α), IL4, IL6, tumour necrosis factor α (TNFα), and basic fibroblast growth factor (FGF). RESULTS—A small but significant population of cells (14.4 ± 12.9%) expressed α actin under standard culture conditions. Upon treatment with TGFβ1 there was a pronounced increase in the number of cells expressing α actin (68.1 ± 5.49%), accompanied by a change in morphology to a myofibroblast-like phenotype. Other cytokines found within the inflamed joint such as IL1, TNFα , IL6, and basic FGF failed to induce α actin expression. However, IL4, which is normally absent or only present at low concentrations in the RA joint had a similar effect to TGFβ1. It was also found that basic FGF inhibited the induction of α actin expression by TGFβ1 and IL4. CONCLUSION—In the presence of TGFβ1 or IL4, fibroblasts derived from synovial tissue or synovial fluid are induced to differentiate into myofibroblast-like cells containing the α smooth muscle form of actin. This differentiation is inhibited by basic FGF. It is suggested that the balance between these particular cytokines may be important in the modulation of fibroblast behaviour, which could have significant effects on joint repair mechanisms and the generation of fibrous tissue within the rheumatoid joint.
Full Text
The Full Text of this article is available as a PDF (184.5 KB).
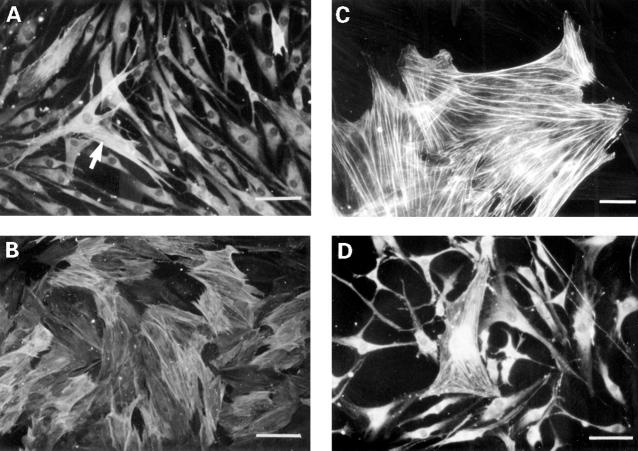
Figure 1 .
Representative photographs to show immunofluorescent staining of α actin in cultures of synovial fibroblasts. (A) In control cultures the majority of cells show no α actin expression and have an elongated, spindle shaped appearance. Occasional cells possess positively stained α actin filaments (arrow). (B) In RA synovial fibroblasts incubated with TGF 1 (5 ng/ml) for three days the majority of cells are stained for α actin. (C) OA synovial fibroblasts incubated with IL4 (1 ng/ml) for three days. Expression of α actin is characterised by the appearance of numerous bundles of α actin filament (stress fibres), and is accompanied by a change to a flattened myofibroblast type morphology. (D) RA synovial fibroblasts incubated with TGF1 (5 ng/ml) in the presence of basic FGF (10 ng/ml ) show no increase in the expression of α actin, and remain mainly spindle shaped, or take on a dendritic morphology. (Bar in (A), (B), (D) represents 4 µm, bar in (C) represents 1 µm).
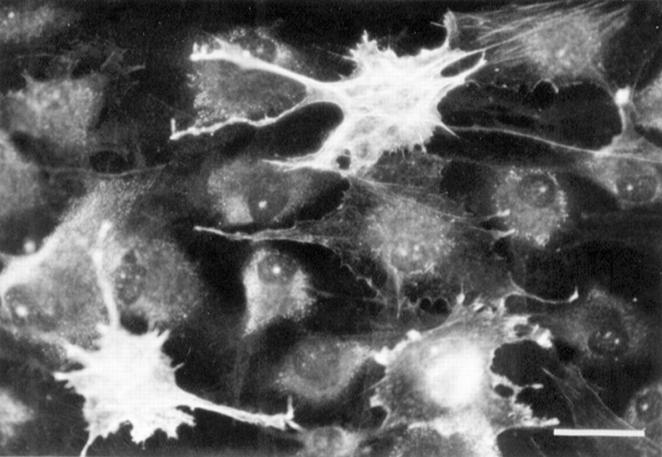
Figure 2 .
Disruption of the α actin cytoskeleton in cells pre-treated with TGFβ1(5 ng/ml) for three days before addition of bFGF (10 ng/ml) for three days. Note that many cells have also taken on a smaller, more dendritic morphology. (Bar represents 2 µm).
Figure 3 .
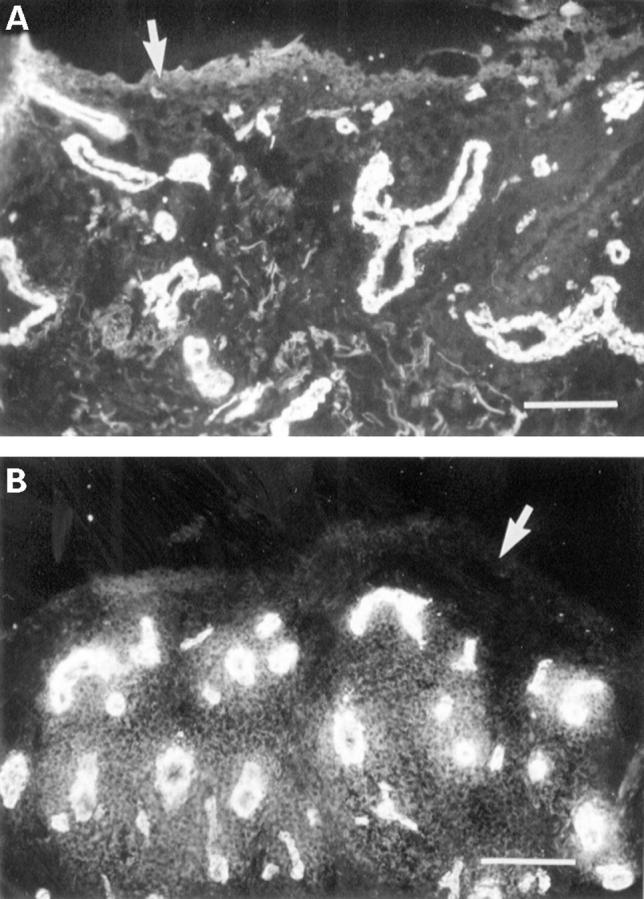
(A) Immunofluorescent staining of α actin in a cryostat section of RA synovium. Little or no staining is present in the lining layer (arrow) but intense staining is found around blood vessels, where smooth muscle cells are situated. (B) α Actin expression in a cryostat section of RA synovium showing a wider zone of staining around the blood vessels. The lining layer is mostly negative (arrow). (Bar represents 8 µm).
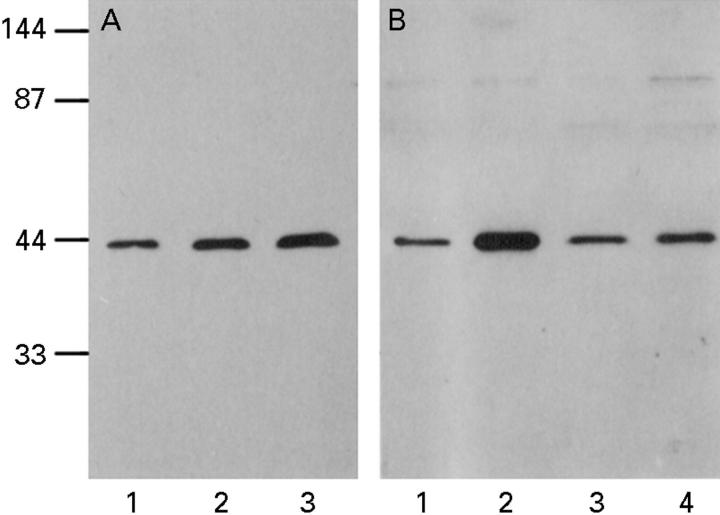
Figure 4 .
Western blots to show α actin expression in synovial fibroblasts with and without cytokine treatment. (A) OA synovial fibroblasts after three days in control medium (lane 1), IL4 (lane 2), TGFβ (lane 3). (B) RA synovial fibroblasts after three days in control medium (lane1), TGFβ1 (lane 2), basic FGF (lane 3), TGFβ1 and bFGF (lane 4). Cytokines were used at the following concentrations ; IL4, 1 ng/ml, TGFβ, 5 ng/ml, bFGF, 10 ng/ml. Molecular weight markers are shown in kilodaltons.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassols A., Massagué J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988 Feb 25;263(6):3039–3045. [PubMed] [Google Scholar]
- Chantry D., Turner M., Abney E., Feldmann M. Modulation of cytokine production by transforming growth factor-beta. J Immunol. 1989 Jun 15;142(12):4295–4300. [PubMed] [Google Scholar]
- Chu C. Q., Field M., Abney E., Zheng R. Q., Allard S., Feldmann M., Maini R. N. Transforming growth factor-beta 1 in rheumatoid synovial membrane and cartilage/pannus junction. Clin Exp Immunol. 1991 Dec;86(3):380–386. doi: 10.1111/j.1365-2249.1991.tb02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarniecki C. W., Chiu H. H., Wong G. H., McCabe S. M., Palladino M. A. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J Immunol. 1988 Jun 15;140(12):4217–4223. [PubMed] [Google Scholar]
- Davidson J. M., Zoia O., Liu J. M. Modulation of transforming growth factor-beta 1 stimulated elastin and collagen production and proliferation in porcine vascular smooth muscle cells and skin fibroblasts by basic fibroblast growth factor, transforming growth factor-alpha, and insulin-like growth factor-I. J Cell Physiol. 1993 Apr;155(1):149–156. doi: 10.1002/jcp.1041550119. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Geinoz A., Gabbiani F., Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993 Jul;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Desmoulière A., Rubbia-Brandt L., Abdiu A., Walz T., Macieira-Coelho A., Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992 Jul;201(1):64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Eddy R. J., Petro J. A., Tomasek J. J. Evidence for the nonmuscle nature of the "myofibroblast" of granulation tissue and hypertropic scar. An immunofluorescence study. Am J Pathol. 1988 Feb;130(2):252–260. [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava R., Olsen N., Keski-Oja J., Moses H., Pincus T. Active and latent forms of transforming growth factor beta activity in synovial effusions. J Exp Med. 1989 Jan 1;169(1):291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Goddard D. H., Grossman S. L., Moore M. E. Autocrine regulation of rheumatoid arthritis synovial cell growth in vitro. Cytokine. 1990 Mar;2(2):149–155. doi: 10.1016/1043-4666(90)90009-i. [DOI] [PubMed] [Google Scholar]
- Goddard D. H., Grossman S. L., Williams W. V., Weiner D. B., Gross J. L., Eidsvoog K., Dasch J. R. Regulation of synovial cell growth. Coexpression of transforming growth factor beta and basic fibroblast growth factor by cultured synovial cells. Arthritis Rheum. 1992 Nov;35(11):1296–1303. doi: 10.1002/art.1780351109. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971 Aug 6;173(3996):548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- Mattey D. L., Evans E., Dawes P. T. The effects of Tenidap on cytokine induced proliferation of human synovial fibroblasts in vitro. Ann Rheum Dis. 1994 Apr;53(4):250–255. doi: 10.1136/ard.53.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Naviliat M., Dupuy d'Angeac A., Sany J., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor beta in rheumatoid synovitis. Arthritis Rheum. 1990 Aug;33(8):1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. Basic and acidic fibroblast growth factors interact with the same cell surface receptors. J Biol Chem. 1986 Apr 25;261(12):5631–5637. [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnov-Jessen L., Petersen O. W. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993 Jun;68(6):696–707. [PubMed] [Google Scholar]
- Taketazu F., Kato M., Gobl A., Ichijo H., ten Dijke P., Itoh J., Kyogoku M., Rönnelid J., Miyazono K., Heldin C. H. Enhanced expression of transforming growth factor-beta s and transforming growth factor-beta type II receptor in the synovial tissues of patients with rheumatoid arthritis. Lab Invest. 1994 May;70(5):620–630. [PubMed] [Google Scholar]