Abstract
OBJECTIVES—To investigate the regulatory roles of interleukin 1β (IL1β), tumour necrosis factor α (TNFα), interferon γ (IFNγ) or transforming growth factor β1 (TGFβ1) on hyaluronan (HA) synthesis by human fibroblastic synovial lining cells. METHODS—Concentrations of HA in culture supernatants of fibroblastic synovial lining cell line (RAMAK-1 cell line) with or without stimulation by IL1β, TNFα, IFNγ or TGFβ1 were measured by sandwich binding protein assay. Levels of HA synthase mRNA of the cells with or without stimulation were detected by reverse transcribed polymerase chain reaction. Molecular weights of HA in the culture supernatants of the cells with or without stimulation were measured using high performance gel permeation liquid chromatography. RESULTS—HA synthesis by the cells was not significantly augmented by TNFα or by IFNγ. It was significantly stimulated by IL1β but inhibited by TGFβ1. Molecular weights of HA in the culture supernatants of the cells were unchanged by stimulation with TNFα. They were remarkably increased by stimulation with IL1β and IFNγ, but reduced with TGFβ1. CONCLUSION—IL1β is an up regulator of HA synthesis, while TGFβ1 is a down regulator. HA production in the synovial lining cells of inflamed joints (for example, rheumatoid arthritis) might be regulated by the balance of these cytokines. Keywords: synovial lining cells; hyaluronan, interleukin 1β; transforming growth factor β1
Full Text
The Full Text of this article is available as a PDF (132.4 KB).
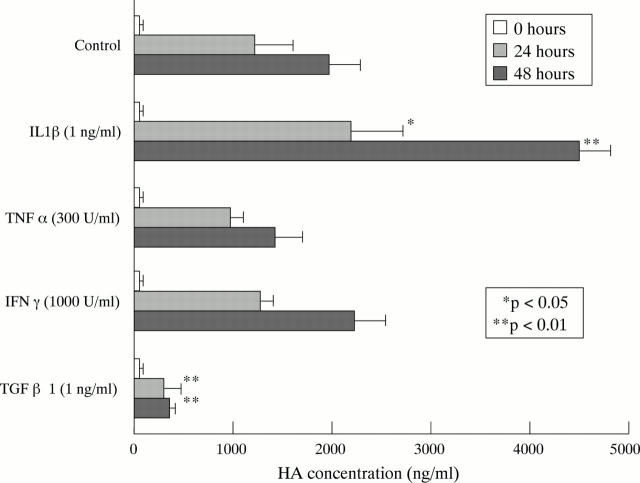
Figure 1 .
HA production by RAMAK-1 cells. RAMAK-1 cells were incubated for 24 or 48 hours in culture dishes with RPMI-1640 medium containing 10% FBS with or without stimulation by IL1β (1 ng/ml), TNFα (300 units/ml), IFNγ (1000 units/ml) or TGFβ1 (1 ng/ml). At the end of culture, the concentration of HA in the supernatants was measured by sandwich binding protein assay.
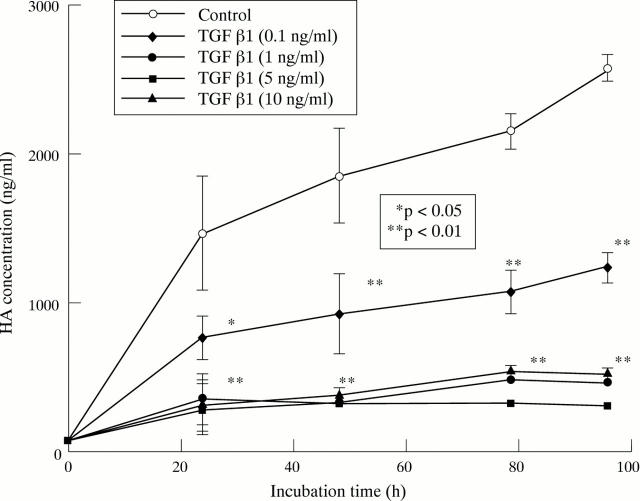
Figure 2 .
Time and dose dependent inhibition of HA production by RAMAK-1 cells cultured with RPMI-1640 medium containing 10% FBS with or without stimulation by TGFβ1. Cells were incubated for up to 96 hours in the presence of TGFβ1. Supernatants were removed for HA determinations by sandwich binding protein assay at 24, 48, 78 or 96 hours. TGFβ1 concentrations indicated were used.
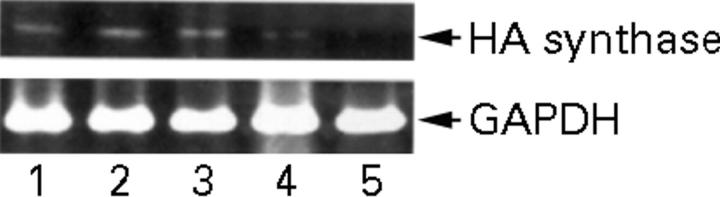
Figure 3 .
HA synthase mRNA of RAMAK-1 cells when stimulated for six hours by IL1β, TNFα, IFNγ or TGFβ1 using RT-PCR. Lane 1, without stimulation; lane 2, with stimulation by IL1β; lane 3, with stimulation by TNFα; lane 4, with stimulation by IFNγ; lane 5, with stimulation by TGFβ1. The primer set amplifies a 548-bp sequence from cDNA transcribed from HA synthase mRNA.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bartolazzi A., Peach R., Aruffo A., Stamenkovic I. Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med. 1994 Jul 1;180(1):53–66. doi: 10.1084/jem.180.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes M. E., Allen J. B., Ogawa Y., Wahl S. M. Transforming growth factor beta 1 suppresses acute and chronic arthritis in experimental animals. J Clin Invest. 1991 Mar;87(3):1108–1113. doi: 10.1172/JCI115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon A. S., Jacoby R. K., Berry H., Hamilton E. B. Clinical trial of intra-articular injection of sodium hyaluronate in patients with osteoarthritis of the knee. Curr Med Res Opin. 1988;11(4):205–213. doi: 10.1185/03007998809114237. [DOI] [PubMed] [Google Scholar]
- Elford P. R., Graeber M., Ohtsu H., Aeberhard M., Legendre B., Wishart W. L., MacKenzie A. R. Induction of swelling, synovial hyperplasia and cartilage proteoglycan loss upon intra-articular injection of transforming growth factor beta-2 in the rabbit. Cytokine. 1992 May;4(3):232–238. doi: 10.1016/1043-4666(92)90061-u. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A., Hällgren R. Circulating hyaluronic acid levels vary with physical activity in healthy subjects and in rheumatoid arthritis patients. Relationship to synovitis mass and morning stiffness. Arthritis Rheum. 1987 Dec;30(12):1333–1338. doi: 10.1002/art.1780301203. [DOI] [PubMed] [Google Scholar]
- Gotoh S., Onaya J., Abe M., Miyazaki K., Hamai A., Horie K., Tokuyasu K. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann Rheum Dis. 1993 Nov;52(11):817–822. doi: 10.1136/ard.52.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grecomoro G., Martorana U., Di Marco C. Intra-articular treatment with sodium hyaluronate in gonarthrosis: a controlled clinical trial versus placebo. Pharmatherapeutica. 1987;5(2):137–141. [PubMed] [Google Scholar]
- Haubeck H. D., Kock R., Fischer D. C., Van de Leur E., Hoffmeister K., Greiling H. Transforming growth factor beta 1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis Rheum. 1995 May;38(5):669–677. doi: 10.1002/art.1780380515. [DOI] [PubMed] [Google Scholar]
- Itano N., Kimata K. Molecular cloning of human hyaluronan synthase. Biochem Biophys Res Commun. 1996 May 24;222(3):816–820. doi: 10.1006/bbrc.1996.0827. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Kitani A., Hara M., Harigai M., Hirose T., Suzuki K., Kawakami M., Hidaka T., Ishizuka T., Kawagoe M. Cytokine regulation of prolyl 4-hydroxylase production in skin fibroblast cultures from patients with systemic sclerosis: contribution to collagen synthesis and fibrosis. J Rheumatol. 1992 Aug;19(8):1195–1201. [PubMed] [Google Scholar]
- Kawakami A., Eguchi K., Matsuoka N., Tsuboi M., Kawabe Y., Aoyagi T., Nagataki S. Inhibition of Fas antigen-mediated apoptosis of rheumatoid synovial cells in vitro by transforming growth factor beta 1. Arthritis Rheum. 1996 Aug;39(8):1267–1276. doi: 10.1002/art.1780390802. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Matsukuma S., Hara M., Hidaka T., Suzuki K., Kitani A., Hiroi S., Ishizuka T., Matsuki Y., Nakamura H. A novel synoviocyte line (RAMAK-1), derived from a patient with rheumatoid arthritis. In Vitro Cell Dev Biol Anim. 1998 Feb;34(2):85–87. doi: 10.1007/s11626-998-0086-x. [DOI] [PubMed] [Google Scholar]
- Kuruvilla A. P., Shah R., Hochwald G. M., Liggitt H. D., Palladino M. A., Thorbecke G. J. Protective effect of transforming growth factor beta 1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Fraser J. R. Hyaluronan. FASEB J. 1992 Apr;6(7):2397–2404. [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Miller D., O'Connor P., Williams J. Use of Na-hyaluronate during intraocular lens implantation in rabbits. Ophthalmic Surg. 1977 Dec;8(6):58–61. [PubMed] [Google Scholar]
- Namiki O., Toyoshima H., Morisaki N. Therapeutic effect of intra-articular injection of high molecular weight hyaluronic acid on osteoarthritis of the knee. Int J Clin Pharmacol Ther Toxicol. 1982 Nov;20(11):501–507. [PubMed] [Google Scholar]
- Oksala O., Salo T., Tammi R., Häkkinen L., Jalkanen M., Inki P., Larjava H. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995 Feb;43(2):125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- Peyron J. G. Intraarticular hyaluronan injections in the treatment of osteoarthritis: state-of-the-art review. J Rheumatol Suppl. 1993 Aug;39:10–15. [PubMed] [Google Scholar]
- Pitsillides A. A., Worrall J. G., Wilkinson L. S., Bayliss M. T., Edwards J. C. Hyaluronan concentration in non-inflamed and rheumatoid synovium. Br J Rheumatol. 1994 Jan;33(1):5–10. doi: 10.1093/rheumatology/33.1.5. [DOI] [PubMed] [Google Scholar]
- Spicer A. P., McDonald J. A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J Biol Chem. 1998 Jan 23;273(4):1923–1932. doi: 10.1074/jbc.273.4.1923. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Asplund T., Yamashita H., Heldin C. H., Heldin P. Stimulation of hyaluronan biosynthesis by platelet-derived growth factor-BB and transforming growth factor-beta 1 involves activation of protein kinase C. Biochem J. 1995 May 1;307(Pt 3):817–821. doi: 10.1042/bj3070817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann D. A., Radin E. L., Nazimiec M., Weisser P. A., Curran N., Lewinnek G. Role of hyaluronic acid in joint lubrication. Ann Rheum Dis. 1974 Jul;33(4):318–326. doi: 10.1136/ard.33.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbecke G. J., Shah R., Leu C. H., Kuruvilla A. P., Hardison A. M., Palladino M. A. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor beta during induction of collagen type II arthritis in mice. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. B., Nguyen H. A., Shizari M., Culty M. CD44 positive macrophages take up hyaluronan during lung development. Dev Biol. 1993 Feb;155(2):324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- Vuorio E., Einola S., Hakkarainen S., Penttinen R. Synthesis of underpolymerized hyaluronic acid by fibroblasts cultured from rheumatoid and non-rheumatoid synovitis. Rheumatol Int. 1982;2(3):97–102. doi: 10.1007/BF00541160. [DOI] [PubMed] [Google Scholar]
- Worrall J. G., Bayliss M. T., Edwards J. C. Morphological localization of hyaluronan in normal and diseased synovium. J Rheumatol. 1991 Oct;18(10):1466–1472. [PubMed] [Google Scholar]