Abstract
OBJECTIVE—To assess if the bonding interlayer between the implant and bone in aseptic loosening of total hip replacement (THR) is qualitatively deteriorated by excessive accumulation of anti-adhesive glycoprotein, tenascin-C. METHODS—Alkaline phosphatase-anti-alkaline phosphatase (APAAP) method was used for immunohistochemical staining of tenascin-C in interface tissue and control synovial tissue. RESULTS—Tenascin-C was found to be a major component of the extracellular matrix at a hitherto unrecognised site, namely the synovial membrane-like interface tissue between implant and bone in aseptic loosening of THR. The overall tenascin-C staining (median score 4.0) was greatly increased in aseptic loosening compared with synovial membrane (median score 2.0; p<0.001) and fibrous capsule (median score 2.0; p<0.001) from primary THR operations. Topological analysis disclosed that tenascin-C was also found at the critical implant-interface and interface-bone surfaces. CONCLUSION—Local tenascin-C expression is increased as a result of a chronic foreign body type reaction associated with excessive cytokine production and tissue injury mediated by microtrauma and neutral endoproteinases. This qualitative and topological deterioration of the bonding interlayer by an increase of anti-adhesive tenascin-C expression may inadvertantly contribute to loosening. Keywords: tenascin; aseptic loosening; total hip replacement
Full Text
The Full Text of this article is available as a PDF (145.7 KB).
Figure 1 .
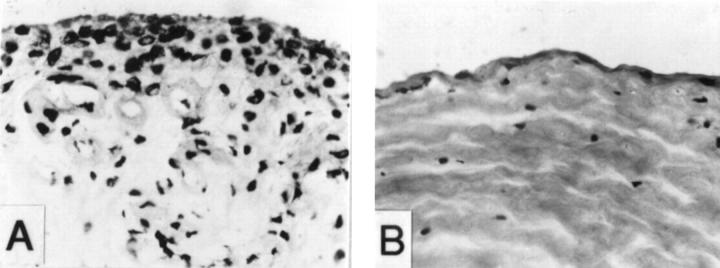
Immunoreactivity for tenascin-C in the synovial membrane-like interface tissue from aseptic loosening of THR and control synovial samples (APAAP staining, original magnification × 250). (A) Immunoreactivity for tenascin-C in the synovial membrane-like interface tissue. Tenascin-C staining was very strong in the synovial lining-like layer (arrows) of interface tissue. This sample/ section also contains intervening matrix, which does not stain for tenascin. (B)Tenascin-C was detected in all fields/extracellular matrix of the synovial membrane-like interface tissue in this revision THR patient. Particularly strong staining was found in the synovial lining-like layer (larger arrows). Heavy deposits of debris (see (C) for verification) were found embedded in the tissue (small arrows). (C) Same section as in (B) photographed with polarised light shows that there were many birefringent polyethylene particles embedded in the tissue (some are marked with arrows). (D) Fibroblasts and their pericellular matrix (arrows) showed usually intense tenascin expression in the synovial membrane-like interface tissue samples, whereas the extracellular collagenous matrix did not stain as strongly (the white intervening areas). Note, that fibroblasts send long and slender extensions, which pass between the collagenous fibres of the connective tissue and would be difficult to identify without tenascin-C staining. (E) Same section as in (D) photographed with polarised light shows polyethylene deposits (some are marked with small arrows). (F) Very weak tenascin-C staining in the control synovial sample from osteoarthritis. (G) For staining control the specific primary IgG2a antibody were replaced with monoclonal IgG with an irrelevant specificity (Aspergillus niger glucose oxidase), but of the same subtype and concentration as the specific primary antibody. Comparison with the (A) confirms the specificity of the staining.
Figure 2 .
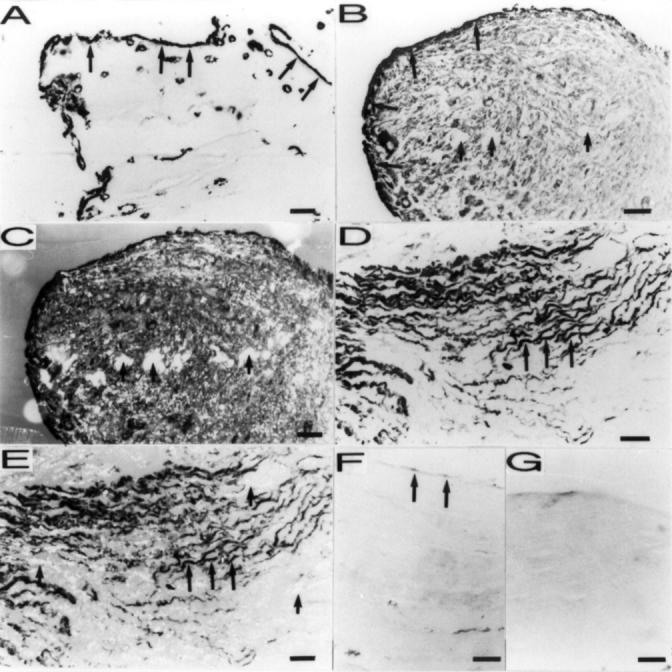
Histological examination of the interface tissue samples with different staining scores (haematoxylin and eosin staining, original magnification × 250). (A) Macrophage-like cells accumulation in a synovial membrane-like interface tissue sample with high staining score (staining score = 4). (B) Matrix fibrosis in the interface tissue sample with lower staining score (staining score = 3).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Regulation of development and differentiation by the extracellular matrix. Development. 1993 Apr;117(4):1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Aukhil I., Joshi P., Yan Y., Erickson H. P. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993 Feb 5;268(4):2542–2553. [PubMed] [Google Scholar]
- Carter D. H., Sloan P., Aaron J. E. Immunolocalization of collagen types I and III, tenascin, and fibronectin in intramembranous bone. J Histochem Cytochem. 1991 May;39(5):599–606. doi: 10.1177/39.5.1707904. [DOI] [PubMed] [Google Scholar]
- Chevalier X., Groult N., Larget-Piet B., Zardi L., Hornebeck W. Tenascin distribution in articular cartilage from normal subjects and from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1994 Jul;37(7):1013–1022. doi: 10.1002/art.1780370706. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Chung C. Y., Murphy-Ullrich J. E., Erickson H. P. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996 Jun;7(6):883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M., Picasso M., Ponassi M., Sun M. Z., Balza E. Tenascin and fibronectin distribution in human normal and pathological synovium. J Rheumatol. 1992 Sep;19(9):1439–1447. [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Aufderheide E. Stimulation of tenascin expression in mesenchyme by epithelial-mesenchymal interactions. Int J Dev Biol. 1989 Mar;33(1):71–79. [PubMed] [Google Scholar]
- Fassbender H. G., Gay S. Synovial processes in rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;76:1–7. doi: 10.3109/03009748809102945. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Martinez-Lacabe V., Virtanen I., Sahlin K. M., Schwartz M. M. Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest. 1992 Jul;67(1):71–79. [PubMed] [Google Scholar]
- Hasegawa T., Seki K., Yang P., Hirose T., Hizawa K., Wada T., Wakabayashi J. Differentiation and proliferative activity in benign and malignant cartilage tumors of bone. Hum Pathol. 1995 Aug;26(8):838–845. doi: 10.1016/0046-8177(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Jiranek W. A., Machado M., Jasty M., Jevsevar D., Wolfe H. J., Goldring S. R., Goldberg M. J., Harris W. H. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993 Jun;75(6):863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Kurvinen H., Takagi M., Michelsson J. E., Eklund K. K., Nordsletten L., Buø L., Aasen A. O., Santavirta S. Interleukin-1 and collagenases around loosening total hip prostheses. Clin Exp Rheumatol. 1996 May-Jun;14(3):255–262. [PubMed] [Google Scholar]
- Konttinen Y. T., Waris V., Xu J. W., Jiranek W. A., Sorsa T., Virtanen I., Santavirta S. Transforming growth factor-beta 1 and 2 in the synovial-like interface membrane between implant and bone in loosening of total hip arthroplasty. J Rheumatol. 1997 Apr;24(4):694–701. [PubMed] [Google Scholar]
- Koukoulis G. K., Gould V. E., Bhattacharyya A., Gould J. E., Howeedy A. A., Virtanen I. Tenascin in normal, reactive, hyperplastic, and neoplastic tissues: biologic and pathologic implications. Hum Pathol. 1991 Jul;22(7):636–643. doi: 10.1016/0046-8177(91)90285-w. [DOI] [PubMed] [Google Scholar]
- Latijnhouwers M. A., Bergers M., Van Bergen B. H., Spruijt K. I., Andriessen M. P., Schalkwijk J. Tenascin expression during wound healing in human skin. J Pathol. 1996 Jan;178(1):30–35. doi: 10.1002/(SICI)1096-9896(199601)178:1<30::AID-PATH442>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Luomanen M., Virtanen I. Distribution of tenascin in healing incision, excision and laser wounds. J Oral Pathol Med. 1993 Jan;22(1):41–45. doi: 10.1111/j.1600-0714.1993.tb00118.x. [DOI] [PubMed] [Google Scholar]
- Mackie E. J., Halfter W., Liverani D. Induction of tenascin in healing wounds. J Cell Biol. 1988 Dec;107(6 Pt 2):2757–2767. doi: 10.1083/jcb.107.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Scott-Burden T., Hahn A. W., Kern F., Bernhardt J., Regenass S., Weller A., Bühler F. R. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol. 1992 Aug;141(2):377–388. [PMC free article] [PubMed] [Google Scholar]
- Mackie E. J., Thesleff I., Chiquet-Ehrismann R. Tenascin is associated with chondrogenic and osteogenic differentiation in vivo and promotes chondrogenesis in vitro. J Cell Biol. 1987 Dec;105(6 Pt 1):2569–2579. doi: 10.1083/jcb.105.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Ladner U., Kriegsmann J., Geiler T., Gay R. E., Gay S. Synovial mesenchymal reaction as a perpetuating principle. Scand J Rheumatol Suppl. 1995;101:115–119. doi: 10.3109/03009749509100912. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pällysaho T., Tervo K., Kivelä T., Virtanen I., Tarkkanen A., Tervo T. Cellular fibronectin and tenascin in an orbital nylon prosthesis removed because of infection caused by Staphylococcus aureus. Graefes Arch Clin Exp Ophthalmol. 1993 Feb;231(2):61–65. doi: 10.1007/BF00920213. [DOI] [PubMed] [Google Scholar]
- Rettig W. J., Erickson H. P., Albino A. P., Garin-Chesa P. Induction of human tenascin (neuronectin) by growth factors and cytokines: cell type-specific signals and signalling pathways. J Cell Sci. 1994 Feb;107(Pt 2):487–497. [PubMed] [Google Scholar]
- Rooney M., Condell D., Quinlan W., Daly L., Whelan A., Feighery C., Bresnihan B. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988 Aug;31(8):956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Salter D. M. Tenascin is increased in cartilage and synovium from arthritic knees. Br J Rheumatol. 1993 Sep;32(9):780–786. doi: 10.1093/rheumatology/32.9.780. [DOI] [PubMed] [Google Scholar]
- Santavirta S., Konttinen Y. T., Bergroth V., Eskola A., Tallroth K., Lindholm T. S. Aggressive granulomatous lesions associated with hip arthroplasty. Immunopathological studies. J Bone Joint Surg Am. 1990 Feb;72(2):252–258. [PubMed] [Google Scholar]
- Santavirta S., Konttinen Y. T., Hoikka V., Eskola A. Immunopathological response to loose cementless acetabular components. J Bone Joint Surg Br. 1991 Jan;73(1):38–42. doi: 10.1302/0301-620X.73B1.1991772. [DOI] [PubMed] [Google Scholar]
- Santavirta S., Sorsa T., Konttinen Y. T., Saari H., Eskola A., Eisen A. Z. Role of mesenchymal collagenase in the loosening of total hip prosthesis. Clin Orthop Relat Res. 1993 May;(290):206–215. [PubMed] [Google Scholar]
- Takagi M., Konttinen Y. T., Kemppinen P., Sorsa T., Tschesche H., Bläser J., Suda A., Santavirta S. Tissue inhibitor of metalloproteinase 1, collagenolytic and gelatinolytic activity in loose hip endoprostheses. J Rheumatol. 1995 Dec;22(12):2285–2290. [PubMed] [Google Scholar]
- Takagi M., Konttinen Y. T., Lindy O., Sorsa T., Kurvinen H., Suda A., Santavirta S. Gelatinase/type IV collagenases in the loosening of total hip replacement endoprostheses. Clin Orthop Relat Res. 1994 Sep;(306):136–144. [PubMed] [Google Scholar]
- Takagi M., Konttinen Y. T., Santavirta S., Sorsa T., Eisen A. Z., Nordsletten L., Suda A. Extracellular matrix metalloproteinases around loose total hip prostheses. Acta Orthop Scand. 1994 Jun;65(3):281–286. doi: 10.3109/17453679408995454. [DOI] [PubMed] [Google Scholar]
- Tiitta O., Wahlström T., Paavonen J., Linnala A., Sharma S., Gould V. E., Virtanen I. Enhanced tenascin expression in cervical and vulvar koilocytotic lesions. Am J Pathol. 1992 Oct;141(4):907–913. [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P., Hammarback J. A., Jenrath D. A., Mackie E. J., Xu Y. Tenascin expression in the mouse: in situ localization and induction in vitro by bFGF. J Cell Sci. 1993 Jan;104(Pt 1):69–76. doi: 10.1242/jcs.104.1.69. [DOI] [PubMed] [Google Scholar]
- Waris V., Xu J. W., Nordsletten L., Sorsa T., Santavirta S., Konttinen Y. T. Basic fibroblast growth factor (bFGF) in the synovial-like membrane around loose total hip prostheses. Scand J Rheumatol. 1996;25(4):257–262. doi: 10.3109/03009749609069995. [DOI] [PubMed] [Google Scholar]
- Wooley P. H., Nasser S., Fitzgerald R. H., Jr The immune response to implant materials in humans. Clin Orthop Relat Res. 1996 May;(326):63–70. doi: 10.1097/00003086-199605000-00008. [DOI] [PubMed] [Google Scholar]
- Xu J. W., Konttinen Y. T., Lassus J., Natah S., Ceponis A., Solovieva S., Aspenberg P., Santavirta S. Tumor necrosis factor-alpha (TNF-alpha) in loosening of total hip replacement (THR). Clin Exp Rheumatol. 1996 Nov-Dec;14(6):643–648. [PubMed] [Google Scholar]