Abstract
OBJECTIVE—The autoantigen p68 is a target of autoantibodies as well as autoreactive T cells with a high specificity in rheumatoid arthritis (RA). The binding characteristics of the autoantibodies to their antigen were now analysed biochemically and cytologically. METHODS—Deglycosylation techniques as well as lectin and sugar competition experiments were performed to p68 to discover if the antibodies detected a glycoepitope. Its antigenicity was investigated applying anti-p68 antibodies derived from RA patients in comparison with polyclonal rabbit anti-p68 antibodies. RESULTS—p68 specific antibodies from RA patients did not to bind to p68 that had been deglycosylated by alkaline β-elimination, O-glycosidase or periodate treatment. In contrast, binding of p68 specific antibodies raised in rabbit was unaffected by either deglycosylation protocol. Furthermore, lectins specific for the carbohydrate N-acetylglucosamine competed with p68 specific antibodies from RA patients for antigen binding. N-acetylglucosamine by itself also competed with patient derived anti-p68 antibodies for p68 binding. Again, rabbit anti-p68 antibodies did not elicit these competitive effects. Applying cytoimmunofluorescence, p68 was present in the cytoplasm or endoplasmic reticulum and also in low abundance on the cell surface. Under heatshock conditions, p68 was detectable in the nucleus. CONCLUSIONS—Autoimmunity to p68 during RA is carried by anti-carbohydrate autoantibodies. The carbohydrate modification of p68 appears to be N-acetylglucosamine, which may reflect the regulation of intracellular localisation of the antigen. It is hypothesised that a shift in glycosylation pattern accompanied by an unphysiological localisation of the antigen could trigger antigenicity of p68 during the pathogenesis of RA. Keywords: carbohydrate epitope; autoantibodies; autoantigen; rheumatoid arthritis
Full Text
The Full Text of this article is available as a PDF (178.6 KB).
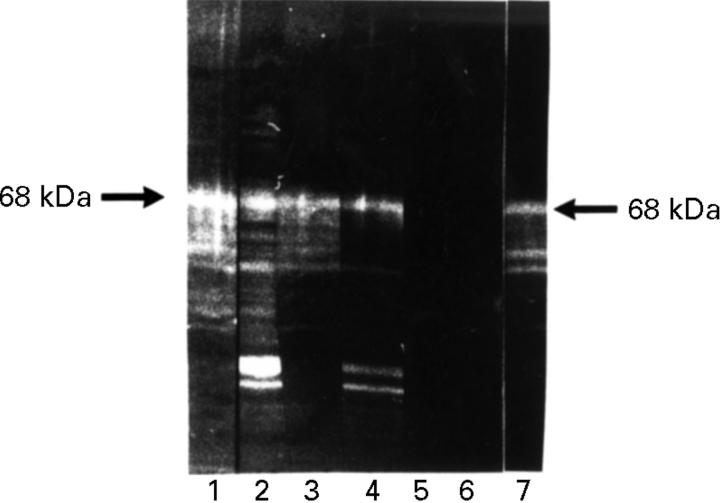
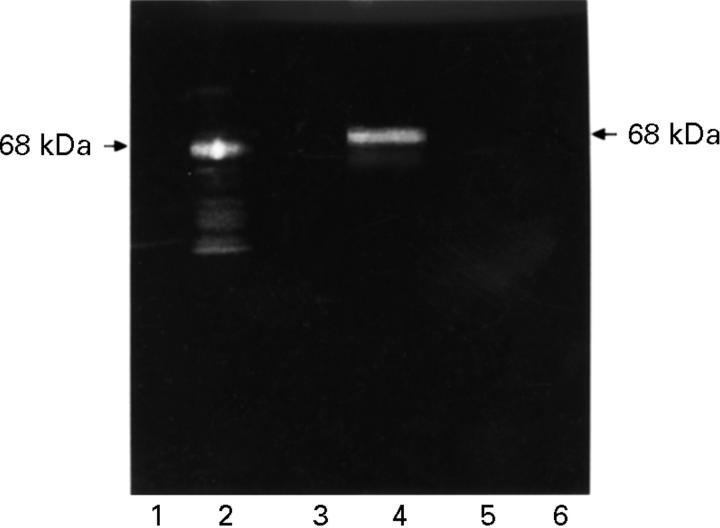
Figure 1 .
Alkaline β-elimination. HeLa protein western blot. Lanes 1 and 2 preincubated in water, 3-5 in 5 mM NaOH, 6-7 in 10 mM NaOH. Subsequent incubation with anti-p68-positive RA serum (2, 4, 6), anti-p68-negative human control serum (5), anti-p68-positive rabbit serum (1, 3, 7) and anti-p68-negative control serum. Detection with FITC conjugated secondary antibody. p68 was detected by the RA serum antibody after incubation with water (2) and with 5 mM NaOH (4), but not after incubation with 10 mM NaOH (6). p68 was detectable by the rabbit serum antibody even after 10 mM NaOH treatment (7).
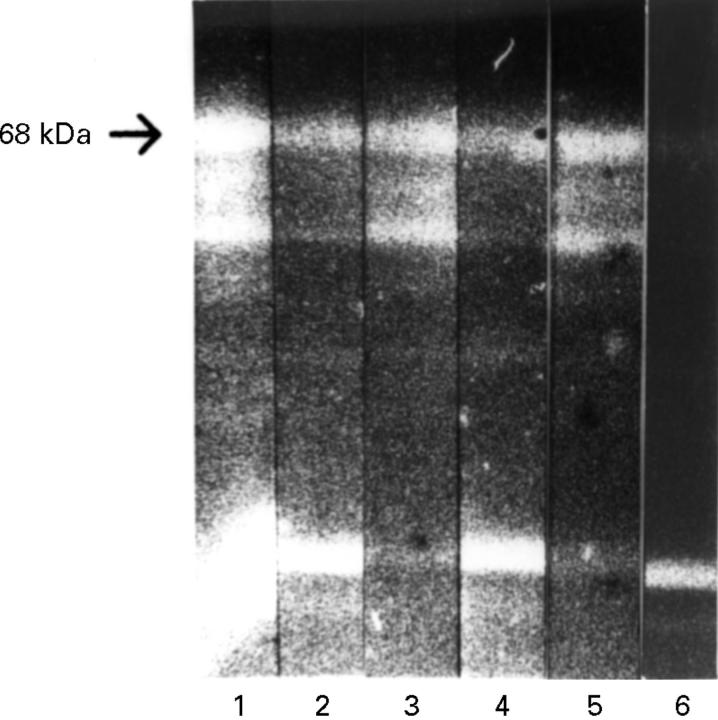
Figure 2 .
O-glycosidase. HeLa protein western blot. Lanes 1 and 2 preincubated with water, 3+4 with N-glycosidase F, 5-6 with O-glycosidase. Subsequent incubation with anti-p68-positive RA serum (2, 4, 6) or anti-p68-positive rabbit serum. Detection with FITC conjugated secondary antibody. p68 was detectable by the RA serum antibody after incubation with water and with N-glycosidase F and not after incubation with O-glycosidase (5). p68 was detectable by the rabbit serum antibody even after treatment with O-glycosidase (6).
Figure 3 .
Periodate treatment. HeLa protein western blot. Lanes 1-3 preincubated with sodium periodate/acetate and 4-6 with sodium acetate for two hours at room temperature. Subsequent incubation with anti-p68-positive RA serum (1 + 4), anti-p68-positive rabbit serum (2 + 5) and anti-p68-negative control serum (3 + 6). Detection with FITC conjugated secondary antibody. After treatment with periodate, p68 was detected by the rabbit (2), but not by the RA serum antibody (1).
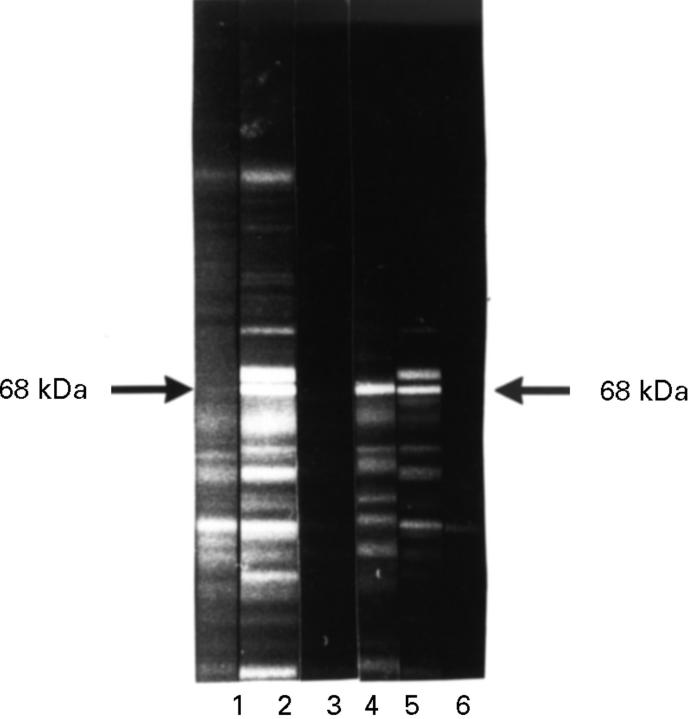
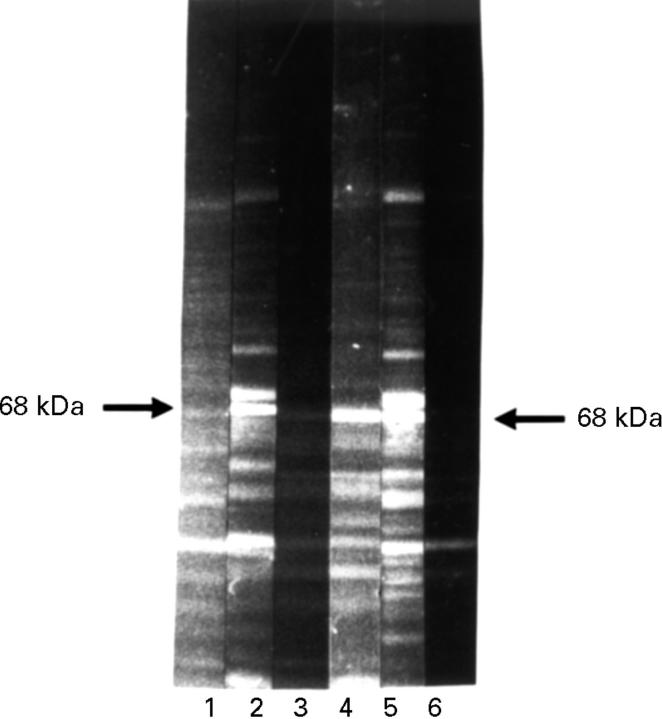
Figure 4 .
Lectin competition. HeLa protein western blot. Lane 1 incubated with anti-p68 negative control serum, 2 with anti-p68 positive RA serum, 3 with anti-p68 positive rabbit serum; 4 + 5 with DBA and 6 +7 with WGA over night. Subsequent incubation with anti-p68 positive RA serum (5 + 7) and with anti-p68 positive rabbit serum (4 + 6). Detection with FITC conjugated secondary antibody. The lectin WGA, but not DBA competed with the RA serum anti-p68 antibody for binding to p68 (7, 5). Neither lectin could compete with the rabbit anti-p68 antibody (6, 4).
Figure 5 .
ConA binding. HeLa protein western blot. Lanes 1 and 2 total HeLa protein, 3+4 ConA column eluate, 5+6 flow through fraction. Incubation with anti-p68-positive RA serum (2, 4, 6) and anti-p68-negative human control serum. Detection with FITC conjugated secondary antibody. p68 could by detected in the eluate, but not in the flow through fraction (4, 6).
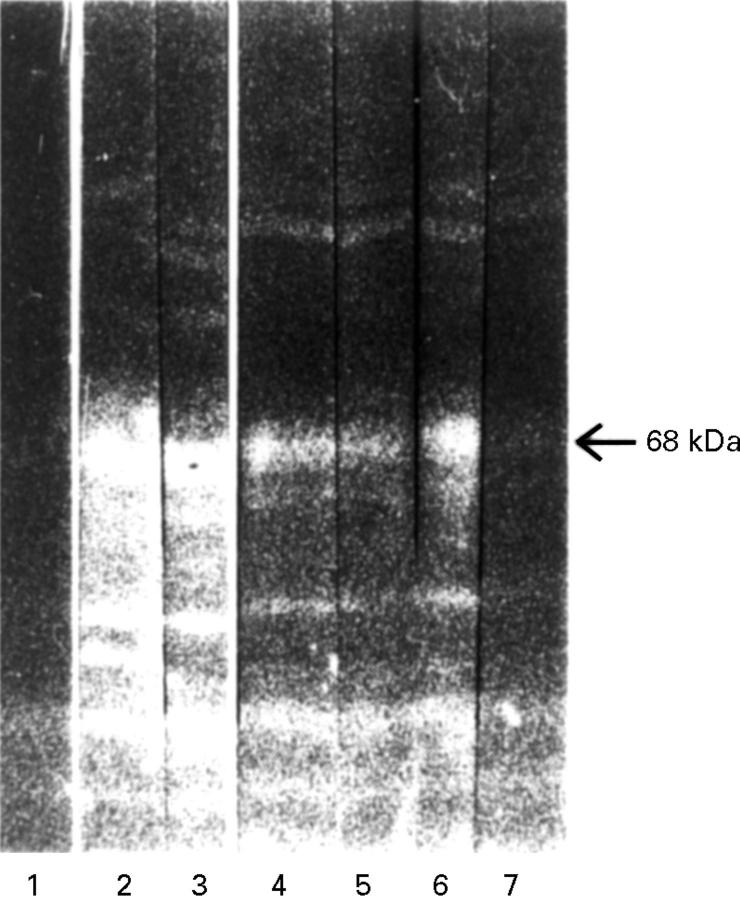
Figure 6 .
Carbohydrate competition. HeLa protein western blot. Preincubation with GlcNAc (1-3) and with water (4-6). Subsequent incubation with anti-p68-positive RA serum (1, 4), anti-p68-positive rabbit serum (2, 5) and anti-p68-negative control serum (3, 6). Detection with FITC conjugated secondary antibody. GlcNAc could compete with the RA, but not with the rabbit serum anti-p68 antibody for binding to p68 (1, 2).
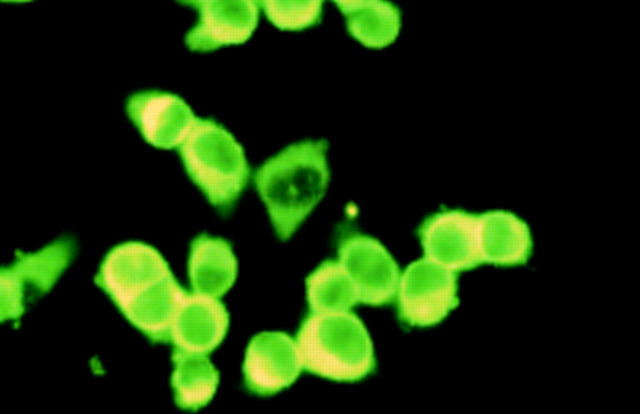
Figure 7 .
Cytoimmunofluorescence on HeLa cells. HeLa cells denatured in 3.5% paraformaldehyde and 0.15% NP40 reacted with human anti-p68 antibodies. Detection using FITC conjugated secondary antibodies. 1 µm in nature equals 3 cm on the picture.
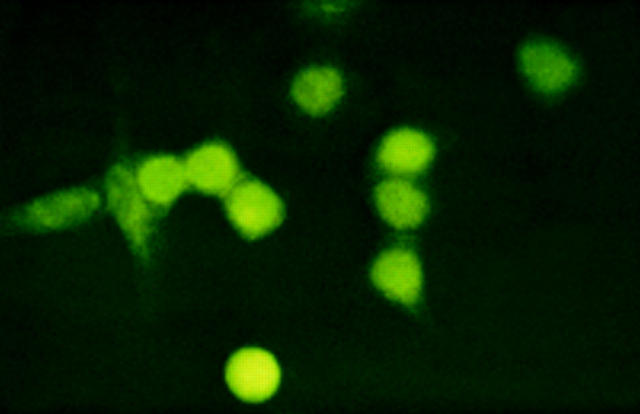
Figure 8 .
Cytoimmunofluorescence on heatshocked HeLa cells. HeLa cells incubated at 42°C for one hour, denatured in 3.5% paraformaldehyde and 0.15% NP40 reacted with human anti-p68 antibodies. Detection using FITC conjugated secondary antibodies. 1 µm in nature equals 3 cm on the picture.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavi A., Axford J. Beta 1,4-galactosyltransferase variations in rheumatoid arthritis. Adv Exp Med Biol. 1995;376:185–192. doi: 10.1007/978-1-4615-1885-3_19. [DOI] [PubMed] [Google Scholar]
- Alavi A., Axford J. Evaluation of beta 1,4-galactosyltransferase in rheumatoid arthritis and its role in the glycosylation network associated with this disease. Glycoconj J. 1995 Jun;12(3):206–210. doi: 10.1007/BF00731321. [DOI] [PubMed] [Google Scholar]
- Almeida I. C., Ferguson M. A., Schenkman S., Travassos L. R. Lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas' disease recognize novel O-linked oligosaccharides on mucin-like glycosyl-phosphatidylinositol-anchored glycoproteins of Trypanosoma cruzi. Biochem J. 1994 Dec 15;304(Pt 3):793–802. doi: 10.1042/bj3040793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bläss S., Haferkamp C., Specker C., Schwochau M., Schneider M., Schneider E. M. Rheumatoid arthritis: autoreactive T cells recognising a novel 68k autoantigen. Ann Rheum Dis. 1997 May;56(5):317–322. doi: 10.1136/ard.56.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläss S., Specker C., Lakomek H. J., Schneider E. M., Schwochau M. Novel 68 kDa autoantigen detected by rheumatoid arthritis specific antibodies. Ann Rheum Dis. 1995 May;54(5):355–360. doi: 10.1136/ard.54.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman K. B., Hutchings P. R., Jeddi P. A., Delves P. J., Rook G. A., Sumar N., Roitt I. M., Lydyard P. M. IgG glycosylation in autoimmune-prone strains of mice. Clin Exp Immunol. 1994 Jan;95(1):103–107. doi: 10.1111/j.1365-2249.1994.tb06022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonagura V. R., Artandi S. E., Davidson A., Randen I., Agostino N., Thompson K., Natvig J. B., Morrison S. L. Mapping studies reveal unique epitopes on IgG recognized by rheumatoid arthritis-derived monoclonal rheumatoid factors. J Immunol. 1993 Oct 1;151(7):3840–3852. [PubMed] [Google Scholar]
- Bond A., Alavi A., Axford J. S., Bourke B. E., Bruckner F. E., Kerr M. A., Maxwell J. D., Tweed K. J., Weldon M. J., Youinou P. A detailed lectin analysis of IgG glycosylation, demonstrating disease specific changes in terminal galactose and N-acetylglucosamine. J Autoimmun. 1997 Feb;10(1):77–85. doi: 10.1006/jaut.1996.0104. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chou T. Y., Dang C. V., Hart G. W. Glycosylation of the c-Myc transactivation domain. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4417–4421. doi: 10.1073/pnas.92.10.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. A., Reason A. J., Morris H. R., Dell A., Iglesias R., Ubeira F. M., Appleton J. A. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994 Oct;4(5):585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- Isenberg D. A. Humoral immunity and glycosylation abnormalities in rheumatoid arthritis. Clin Exp Rheumatol. 1995 Sep-Oct;13 (Suppl 12):S17–S20. [PubMed] [Google Scholar]
- Kumpel B. M., Rademacher T. W., Rook G. A., Williams P. J., Wilson I. B. Galactosylation of human IgG monoclonal anti-D produced by EBV-transformed B-lymphoblastoid cell lines is dependent on culture method and affects Fc receptor-mediated functional activity. Hum Antibodies Hybridomas. 1994;5(3-4):143–151. [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Prokopová A., Kéry V., Stancíková M., Grimová J., Capek P., Sandula J., Orviský E. Methyl-alpha-D-mannopyranoside, mannooligosaccharides and yeast mannans inhibit development of rat adjuvant arthritis. J Rheumatol. 1993 Apr;20(4):673–677. [PubMed] [Google Scholar]
- Rademacher T. W., Williams P., Dwek R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Williams P., Dwek R. A. Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6123–6127. doi: 10.1073/pnas.91.13.6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquemore E. P., Chevrier M. R., Cotter R. J., Hart G. W. Dynamic O-GlcNAcylation of the small heat shock protein alpha B-crystallin. Biochemistry. 1996 Mar 19;35(11):3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- Shikhman A. R., Cunningham M. W. Immunological mimicry between N-acetyl-beta-D-glucosamine and cytokeratin peptides. Evidence for a microbially driven anti-keratin antibody response. J Immunol. 1994 May 1;152(9):4375–4387. [PubMed] [Google Scholar]
- Shikhman A. R., Greenspan N. S., Cunningham M. W. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-D-glucosamine. J Immunol. 1993 Oct 1;151(7):3902–3913. [PubMed] [Google Scholar]
- Shikhman A. R., Greenspan N. S., Cunningham M. W. Cytokeratin peptide SFGSGFGGGY mimics N-acetyl-beta-D-glucosamine in reaction with antibodies and lectins, and induces in vivo anti-carbohydrate antibody response. J Immunol. 1994 Dec 15;153(12):5593–5606. [PubMed] [Google Scholar]