Abstract
OBJECTIVES—To investigate articular cartilage collagen network, thickness of birefringent cartilage zones, and glycosaminoglycan concentration in macroscopically normal looking knee joint cartilage of young beagles subjected to experimental slowly progressive osteoarthritis (OA). METHODS—OA was induced by a tibial 30° valgus osteotomy in 15 female beagles at the age of 3 months. Fifteen sisters were controls. Cartilage specimens were collected seven (Group 1) and 18 months (Group 2) postoperatively. Collagen induced optical path difference and cartilage zone thickness measurements were determined from histological sections of articular cartilage with smooth and intact surface by computer assisted quantitative polarised light microscopy. Volume density of cartilage collagen fibrils was determined by image analysis from transmission electron micrographs and content of glycosaminoglycans by quantitative digital densitometry from histological sections. Results—In the superficial zone of the lateral tibial and femoral cartilage, the collagen induced optical path difference (birefringence) decreased by 19 to 71% (p < 0.05) seven months postoperatively. This suggests that severe superficial collagen fibril network deterioration took place, as 18 months postoperatively, macroscopic and microscopic OA was present in many cartilage areas. Thickness of the uncalcified cartilage increased while the superficial zone became thinner in the same sites. In operated dogs, glycosaminoglycan content first increased (Group 1) in the lateral tibial condyle and then decreased (Group 2) (p < 0.05). Conclusion—In this OA model, derangement of the superficial zone collagen network was the probable reason for birefringence reduction. This change occurred well before macroscopic OA. Keywords: cartilage; birefringence
Full Text
The Full Text of this article is available as a PDF (191.9 KB).
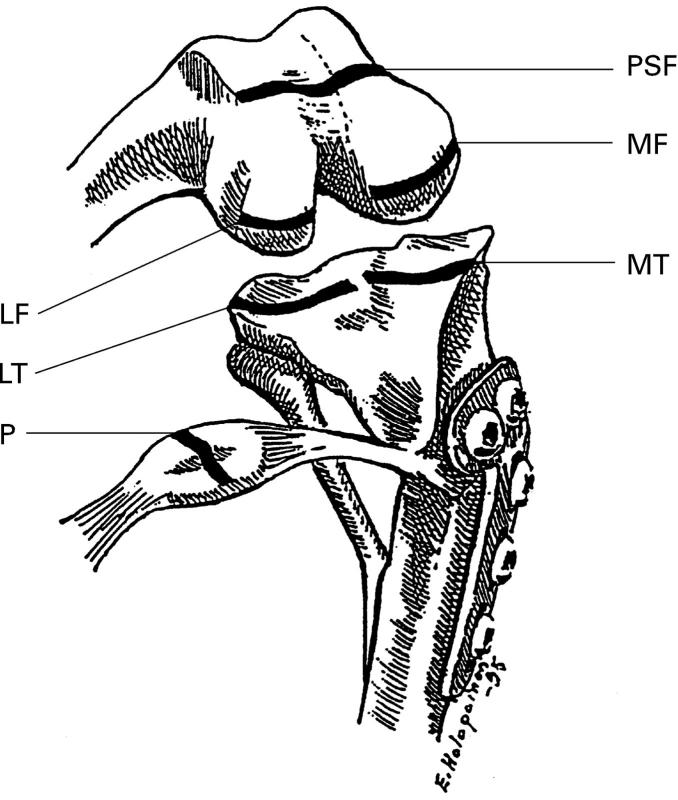
Figure 1 .
Sites of the polarised light microscopy analysis in the right knee (stifle) joint of valgus osteotomised dogs and controls. PSF = patellar surface of femur, P = patella, LF = lateral condyle of femur, MF = medial condyle of femur, LT = lateral condyle of tibia and MT = medial condyle of tibia. Samples from LF and LT were taken also for computer based digital densitometry and from LT for transmission electron microscopic analysis.
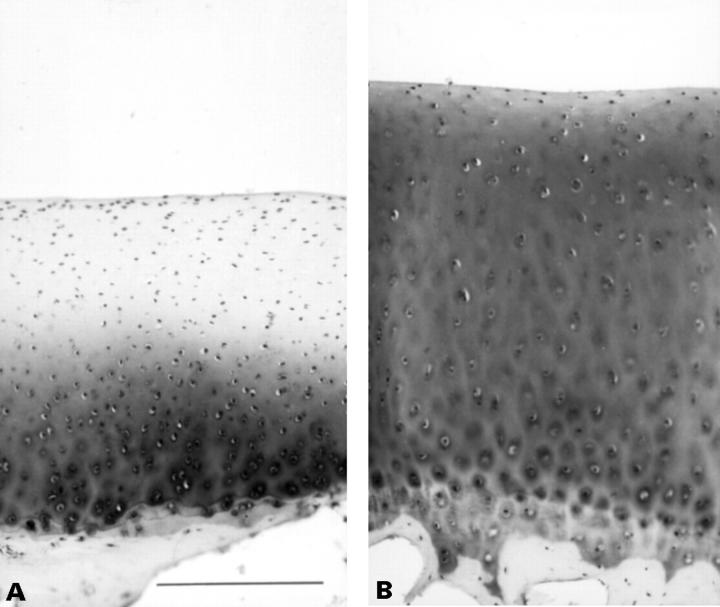
Figure 2 .
Polarised light micrographs taken from unstained sections of the articular cartilage from the lateral condyle of tibia (LT) from a valgus operated dog (seven months postoperatively) (A) and a littermate control (B). Birefringence in the superficial zone of the operated dog is markedly decreased compared with control. On the other hand, in the deep zone birefringence is increased in the operated animal (scale 125 µm).
Figure 3 .
A photomicrograph taken from the lateral tibial (LT) condyle of the right knee joint 18 months after valgus osteotomy. In the superficial and intermediate zones of articular cartilage, severe reduction in safranin O staining is evident. In this case, the thickness of uncalcified and calcified cartilage is reduced (A). A photomicrograph from the littermate control dog showing normal articular cartilage from the same area (B) (safranin O, fast green and iron haematoxylin, scale 250 µm).
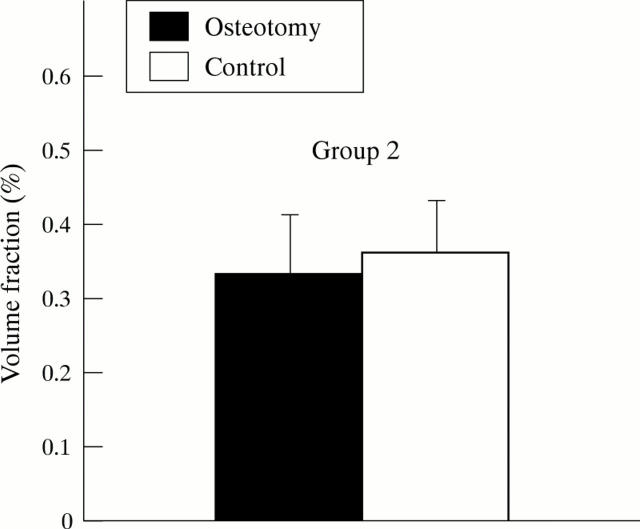
Figure 4 .
Volume fraction (%) of collagen from the superficial zone of articular cartilage from the lateral condyle of tibia (LT) in operated and control dogs.
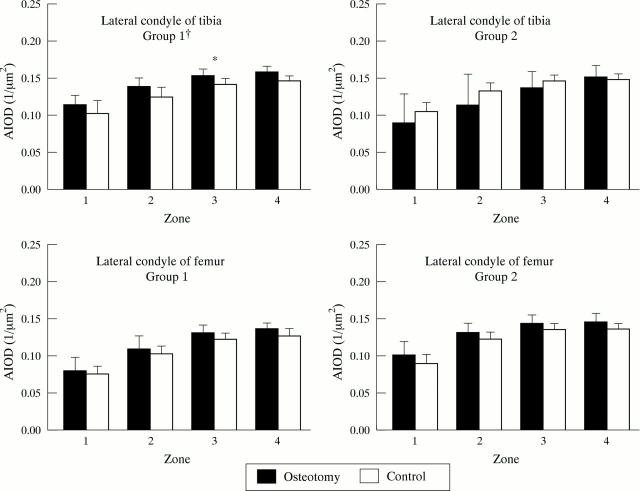
Figure 5 .
Histogram of the area integrated optical density (AIOD 1/µm2) of safranin O staining in articular cartilage from the lateral condyle of tibia (LT) and lateral condyle of femur (LF) divided to four zones 7 (Group 1) and 18 months (Group 2) postoperatively in valgus operated and control dogs. * p < 0.05 versus control, by Wilcoxon matched pairs signed ranks test. † p < 0.05 Group 1 versus Group 2, by Mann-Whitney U test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. E., Brandt K. D. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. J Rheumatol. 1991 Mar;18(3):428–435. [PubMed] [Google Scholar]
- Adams M. E. Cartilage hypertrophy following canine anterior cruciate ligament transection differs among different areas of the joint. J Rheumatol. 1989 Jun;16(6):818–824. [PubMed] [Google Scholar]
- Afzelius B. A. Section staining for electron microscopy using tannic acid as a mordant: a simple method for visualization of glycogen and collagen. Microsc Res Tech. 1992 Mar 1;21(1):65–72. doi: 10.1002/jemt.1070210110. [DOI] [PubMed] [Google Scholar]
- Arokoski J. P., Hyttinen M. M., Lapveteläinen T., Takács P., Kosztáczky B., Módis L., Kovanen V., Helminen H. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann Rheum Dis. 1996 Apr;55(4):253–264. doi: 10.1136/ard.55.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arokoski J., Jurvelin J., Kiviranta I., Tammi M., Helminen H. J. Softening of the lateral condyle articular cartilage in the canine knee joint after long distance (up to 40 km/day) running training lasting one year. Int J Sports Med. 1994 Jul;15(5):254–260. doi: 10.1055/s-2007-1021056. [DOI] [PubMed] [Google Scholar]
- Arokoski J., Kiviranta I., Jurvelin J., Tammi M., Helminen H. J. Long-distance running causes site-dependent decrease of cartilage glycosaminoglycan content in the knee joints of beagle dogs. Arthritis Rheum. 1993 Oct;36(10):1451–1459. doi: 10.1002/art.1780361018. [DOI] [PubMed] [Google Scholar]
- Bader D. L., Kempson G. E., Egan J., Gilbey W., Barrett A. J. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta. 1992 Apr 22;1116(2):147–154. doi: 10.1016/0304-4165(92)90111-7. [DOI] [PubMed] [Google Scholar]
- Bentley G. Articular cartilage changes in chondromalacia patellae. J Bone Joint Surg Br. 1985 Nov;67(5):769–774. doi: 10.1302/0301-620X.67B5.4055879. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Myers S. L., Burr D., Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991 Dec;34(12):1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- Byers P. D., Maroudase A., Oztop F., Stockwell R. A., Venn M. F. Histological and biochemical studies on cartilage from osteoarthrotic femoral heads with special reference to surface characteristics. Connect Tissue Res. 1977;5(1):41–49. doi: 10.3109/03008207709152611. [DOI] [PubMed] [Google Scholar]
- Dunham J., Shackleton D. R., Nahir A. M., Billingham M. E., Bitensky L., Chayen J., Muir I. H. Altered orientation of glycosaminoglycans and cellular changes in the tibial cartilage in the first two weeks of experimental canine osteoarthritis. J Orthop Res. 1985;3(3):258–268. doi: 10.1002/jor.1100030302. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., McDevitt C. A., Billingham M. E., Muir H. Biosynthesis of collagen and other matrix proteins by articular cartilage in experimental osteoarthrosis. Biochem J. 1980 Jun 15;188(3):823–837. doi: 10.1042/bj1880823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. B., Lee J., Smith R. L., Csongradi J. C., Fornasier V. L. Mechanical overload of a single compartment induces early degenerative changes in the rabbit knee: a preliminary study. J Invest Surg. 1991;4(2):161–170. doi: 10.3109/08941939109140776. [DOI] [PubMed] [Google Scholar]
- Guilak F., Ratcliffe A., Lane N., Rosenwasser M. P., Mow V. C. Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res. 1994 Jul;12(4):474–484. doi: 10.1002/jor.1100120404. [DOI] [PubMed] [Google Scholar]
- Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981 Dec;9(6):489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Poole A. R. Degenerative changes in dog articular cartilage induced by a unilateral tibial valgus osteotomy. Exp Pathol. 1988;33(3):145–164. doi: 10.1016/s0232-1513(88)80061-6. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Pollard C., Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta. 1973 Feb 28;297(2):456–472. doi: 10.1016/0304-4165(73)90093-7. [DOI] [PubMed] [Google Scholar]
- Király K., Lammi M., Arokoski J., Lapveteläinen T., Tammi M., Helminen H., Kiviranta I. Safranin O reduces loss of glycosaminoglycans from bovine articular cartilage during histological specimen preparation. Histochem J. 1996 Feb;28(2):99–107. doi: 10.1007/BF02331414. [DOI] [PubMed] [Google Scholar]
- Király K., Lapveteläinen T., Arokoski J., Törrönen K., Módis L., Kiviranta I., Helminen H. J. Application of selected cationic dyes for the semiquantitative estimation of glycosaminoglycans in histological sections of articular cartilage by microspectrophotometry. Histochem J. 1996 Aug;28(8):577–590. doi: 10.1007/BF02331378. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Jurvelin J., Tammi M., Sämänen A. M., Helminen H. J. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82(3):249–255. doi: 10.1007/BF00501401. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Sämänen A. M., Helminen H. J. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6(2):188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Lippiello L., Hall D., Mankin H. J. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977 Apr;59(4):593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovász G., Llinás A., Benya P., Bodey B., McKellop H. A., Luck J. V., Jr, Sarmiento A. Effects of valgus tibial angulation on cartilage degeneration in the rabbit knee. J Orthop Res. 1995 Nov;13(6):846–853. doi: 10.1002/jor.1100130607. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Maroudas A. I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976 Apr 29;260(5554):808–809. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Maroudas A., Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977 Oct;36(5):399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br. 1976 Feb;58(1):94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Meachim G., Osborne G. V. Repair at the femoral articular surface in osteo-arthritis of the hip. J Pathol. 1970 Sep;102(1):1–8. doi: 10.1002/path.1711020102. [DOI] [PubMed] [Google Scholar]
- Mizrahi J., Maroudas A., Lanir Y., Ziv I., Webber T. J. The "instantaneous" deformation of cartilage: effects of collagen fiber orientation and osmotic stress. Biorheology. 1986;23(4):311–330. doi: 10.3233/bir-1986-23402. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Ratcliffe A., Poole A. R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- Muir H. Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans. 1983 Dec;11(6):613–622. doi: 10.1042/bst0110613. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford C. R., Gardner D. L., O'Connor P. Ultrastructural changes in dog femoral condylar cartilage following anterior cruciate ligament section. J Anat. 1983 Dec;137(Pt 4):653–663. [PMC free article] [PubMed] [Google Scholar]
- Panula H. E., Helminen H. J., Kiviranta I. Slowly progressive osteoarthritis after tibial valgus osteotomy in young beagle dogs. Clin Orthop Relat Res. 1997 Oct;(343):192–202. [PubMed] [Google Scholar]
- Pond M. J., Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973 Jul;32(4):387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann I. Experimental osteoarthritis of the knee in rabbits induced by alteration of the load-bearing. Acta Orthop Scand. 1973;44(4):496–504. doi: 10.3109/17453677308989085. [DOI] [PubMed] [Google Scholar]
- Sandy J. D., Adams M. E., Billingham M. E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984 Apr;27(4):388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- Schmidt M. B., Mow V. C., Chun L. E., Eyre D. R. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990 May;8(3):353–363. doi: 10.1002/jor.1100080307. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A., Billingham M. E., Muir H. Ultrastructural changes in articular cartilage after experimental section of the anterior cruciate ligament of the dog knee. J Anat. 1983 Mar;136(Pt 2):425–439. [PMC free article] [PubMed] [Google Scholar]
- Vignon E., Arlot M., Hartmann D., Moyen B., Ville G. Hypertrophic repair of articular cartilage in experimental osteoarthrosis. Ann Rheum Dis. 1983 Feb;42(1):82–88. doi: 10.1136/ard.42.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. D., Burr D. B., Boyd R. D., Radin E. L. Bone and cartilage changes following experimental varus or valgus tibial angulation. J Orthop Res. 1990 Jul;8(4):572–585. doi: 10.1002/jor.1100080414. [DOI] [PubMed] [Google Scholar]
- Wu J. J., Lark M. W., Chun L. E., Eyre D. R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991 Mar 25;266(9):5625–5628. [PubMed] [Google Scholar]