Abstract
OBJECTIVE—To clarify the effect of pressure on the expressions of proteoglycan core protein and metabolism related cytokines in a chondrocyte-like cell line, HCS-2/8. METHODS—HCS-2/8 cells were exposed to 1, 5, 10, or 50 MPa of hydrostatic pressure (HP) for two hours, and mRNA expressions of interleukin 6 (IL6) and tumour necrosis factor α (TNFα) were examined by using reverse transcription-polymerase chain reaction (RT-PCR) method with specific primer sets; and mRNA of proteoglycan core protein, stromelysin, and tissue inhibitor of metalloproteinase 1 (TIMP1) were measured with northern blotting. RESULTS—HP exposure caused temporal morphological changes of the cells, but did not affect cellular viability. IL6 and TNFα mRNA expressions were not observed in the control cells under the atmospheric pressure, whereas in the cells treated with HP, pressure dependent enhancement of IL6 mRNA expression was observed between 30 minutes and four hours after the HP release. TNFα mRNA expression also increased 30 minutes after the exposure to 50 MPa of HP and disappeared four hours later. Proteoglycan core protein mRNA levels increased between 30 minutes and four hours after the exposure to 1 or 5 MPa of HP, whereas the levels decreased after 10 or 50 MPa of HP. Stromelysin and TIMP1 mRNA signals did not respond to HP. CONCLUSIONS—HP at excessively high levels induced IL6 and TNFα expression and reduced the expression of proteoglycan core protein, while physiological levels of HP increased the expression of proteoglycan core protein. These findings are important when considering the pathology of osteoarthritis. Keywords: chondrocytes, tumour necrosis factor α; interleukin 6; mRNA
Full Text
The Full Text of this article is available as a PDF (198.3 KB).
Figure 1 .
Morphological changes of HCS-2/8 after exposure to HP. Phase contrast photomicrograms of cells cultured in DMEM containing 10% FBS after HP exposure. (A) Control cells under atmospheric pressure. (B) Thirty minutes after applying 50 MPa of HP. (C) Twenty four hours after applying 50 MPa of HP. (D) Thirty minutes after applying 5 MPa of HP. Bar = 100 µm.
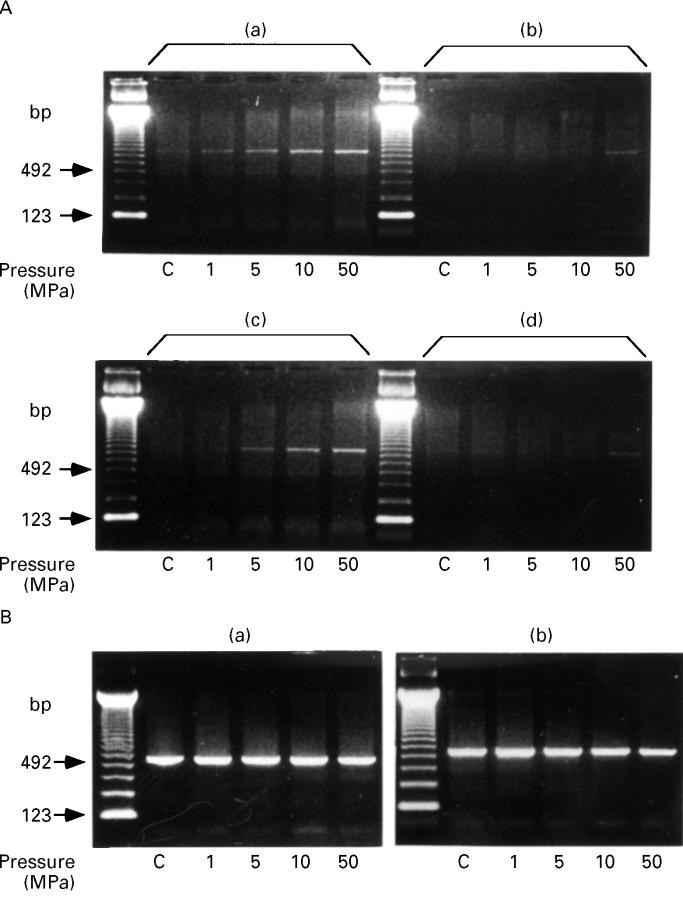
Figure 2 .
Pressure dependent enhancement of IL6 mRNA expression. (A) IL6 RT-PCR products from the cells at 30 minutes (a) and (b) and four hours (c) and (d) after HP exposure. Two point five microgram (a) and (c) and 1 µg (b) and (d) of total RNA were reverse transcribed in 10 µl reaction mixture. (B) β actin RT-PCR products from the cells at 30 minutes (a) and two hours (b), which confirm cDNA isolation. One microgram of total RNA was reverse transcribed in 10 µl reaction mixture. C: control cells treated under the atmospheric pressure.
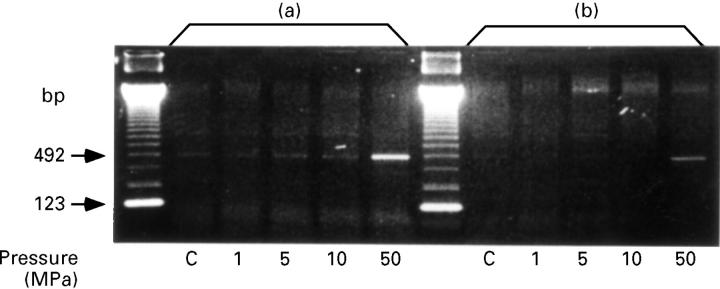
Figure 3 .
Induction of TNFα mRNA expression after HP exposure. The cells at 30 minutes after the exposure to 50 MPa of HP expressed TNFα mRNA. Two point five microgram (a) and 1 µg (b) of total RNA were reverse transcribed in 10 µl reaction mixture. No TNFα mRNA expression was detected in the cells between four and eight hours after HP exposure. C: control cells treated under the atmospheric pressure.
Figure 4 .
Effect of HP exposure on proteoglycan core protein mRNA expression. Cells were exposed to 1, 5, 10 or 50 MPa of HP for two hours, and total RNA was extracted at 30 minutes (A) and four hours (B) after the release of HP. Equal amounts of total RNA were fractionated by gel electrophoresis, transferred to a nylon membrane, and hybridised to RNA probes. For quantification, the signals were measured by using a densitometor. A photograph of gel staining (right side) shows equal amounts of RNA were applied. β actin mRNA expression is also shown. C: control cells treated under the atmospheric pressure.
Figure 5 .
Stromelysin and TIMP1 mRNA signals in HCS-2/8 cells after HP exposure. Representative signals at four hours after HP exposure are shown. C: control cells treated under the atmospheric pressure.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afoke N. Y., Byers P. D., Hutton W. C. Contact pressures in the human hip joint. J Bone Joint Surg Br. 1987 Aug;69(4):536–541. doi: 10.1302/0301-620X.69B4.3611154. [DOI] [PubMed] [Google Scholar]
- Albino A. P., Davis B. M., Nanus D. M. Induction of growth factor RNA expression in human malignant melanoma: markers of transformation. Cancer Res. 1991 Sep 15;51(18):4815–4820. [PubMed] [Google Scholar]
- Arai Y., Kubo T., Kobayashi K., Takeshita K., Takahashi K., Ikeda T., Imanishi J., Takigawa M., Hirasawa Y. Adenovirus vector-mediated gene transduction to chondrocytes: in vitro evaluation of therapeutic efficacy of transforming growth factor-beta 1 and heat shock protein 70 gene transduction. J Rheumatol. 1997 Sep;24(9):1787–1795. [PubMed] [Google Scholar]
- Bagchi T., Larson D. E., Sells B. H. Cytoskeletal association of muscle-specific mRNAs in differentiating L6 rat myoblasts. Exp Cell Res. 1987 Jan;168(1):160–172. doi: 10.1016/0014-4827(87)90425-3. [DOI] [PubMed] [Google Scholar]
- Broom N. D., Myers D. B. A study of the structural response of wet hyaline cartilage to various loading situations. Connect Tissue Res. 1980;7(4):227–237. doi: 10.3109/03008208009152358. [DOI] [PubMed] [Google Scholar]
- Buschmann M. D., Gluzband Y. A., Grodzinsky A. J., Hunziker E. B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995 Apr;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cs-Szabó G., Melching L. I., Roughley P. J., Glant T. T. Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum. 1997 Jun;40(6):1037–1045. doi: 10.1002/art.1780400607. [DOI] [PubMed] [Google Scholar]
- De Witt M. T., Handley C. J., Oakes B. W., Lowther D. A. In vitro response of chondrocytes to mechanical loading. The effect of short term mechanical tension. Connect Tissue Res. 1984;12(2):97–109. doi: 10.3109/03008208408992775. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibb W., Morild E., Laerum O. D. Effects of high hydrostatic pressure on normal and neoplastic rat cells in culture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;38(2):169–176. doi: 10.1007/BF02892812. [DOI] [PubMed] [Google Scholar]
- Dieppe P., Kirwan J. The localization of osteoarthritis. Br J Rheumatol. 1994 Mar;33(3):201–203. doi: 10.1093/rheumatology/33.3.201. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Ornelles D. A., Penman S. Association of RNA with the cytoskeleton and the nuclear matrix. J Cell Sci Suppl. 1986;5:99–119. doi: 10.1242/jcs.1986.supplement_5.6. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Günther M., Haubeck H. D., van de Leur E., Bläser J., Bender S., Gütgemann I., Fischer D. C., Tschesche H., Greiling H., Heinrich P. C. Transforming growth factor beta 1 regulates tissue inhibitor of metalloproteinases-1 expression in differentiated human articular chondrocytes. Arthritis Rheum. 1994 Mar;37(3):395–405. doi: 10.1002/art.1780370314. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Urban J. P., Gehl K. A. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991 Jan;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- Haskin C., Cameron I., Athanasiou K. Physiological levels of hydrostatic pressure alter morphology and organization of cytoskeletal and adhesion proteins in MG-63 osteosarcoma cells. Biochem Cell Biol. 1993 Jan-Feb;71(1-2):27–35. doi: 10.1139/o93-005. [DOI] [PubMed] [Google Scholar]
- Hoch D. H., Grodzinsky A. J., Koob T. J., Albert M. L., Eyre D. R. Early changes in material properties of rabbit articular cartilage after meniscectomy. J Orthop Res. 1983;1(1):4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- Hodge W. A., Fijan R. S., Carlson K. L., Burgess R. G., Harris W. H., Mann R. W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986 May;83(9):2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermann J. R., Kincaid S. A., Rumph P. F., Baird D. K., Visco D. M. Tumor necrosis factor-alpha (TNF-alpha) in canine osteoarthritis: Immunolocalization of TNF-alpha, stromelysin and TNF receptors in canine osteoarthritic cartilage. Osteoarthritis Cartilage. 1996 Mar;4(1):23–34. doi: 10.1016/s1063-4584(96)80004-5. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Ohgitani E., Tanaka Y., Kita M., Imanishi J. Herpes simplex virus-induced expression of 70 kDa heat shock protein (HSP70) requires early protein synthesis but not viral DNA replication. Microbiol Immunol. 1994;38(4):321–325. doi: 10.1111/j.1348-0421.1994.tb01785.x. [DOI] [PubMed] [Google Scholar]
- Kouri J. B., Jiménez S. A., Quintero M., Chico A. Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage. 1996 Jun;4(2):111–125. doi: 10.1016/s1063-4584(05)80320-6. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Intracellular localization of messenger RNAs for cytoskeletal proteins. Cell. 1986 May 9;45(3):407–415. doi: 10.1016/0092-8674(86)90326-0. [DOI] [PubMed] [Google Scholar]
- Lippiello L., Kaye C., Neumata T., Mankin H. J. In vitro metabolic response of articular cartilage segments to low levels of hydrostatic pressure. Connect Tissue Res. 1985;13(2):99–107. doi: 10.3109/03008208509152388. [DOI] [PubMed] [Google Scholar]
- Lotz M., Guerne P. A. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J Biol Chem. 1991 Feb 5;266(4):2017–2020. [PubMed] [Google Scholar]
- Mohtai M., Gupta M. K., Donlon B., Ellison B., Cooke J., Gibbons G., Schurman D. J., Smith R. L. Expression of interleukin-6 in osteoarthritic chondrocytes and effects of fluid-induced shear on this expression in normal human chondrocytes in vitro. J Orthop Res. 1996 Jan;14(1):67–73. doi: 10.1002/jor.1100140112. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Holmes M. H., Lai W. M. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Newman P., Watt F. M. Influence of cytochalasin D-induced changes in cell shape on proteoglycan synthesis by cultured articular chondrocytes. Exp Cell Res. 1988 Oct;178(2):199–210. doi: 10.1016/0014-4827(88)90391-6. [DOI] [PubMed] [Google Scholar]
- Parkkinen J. J., Lammi M. J., Inkinen R., Jortikka M., Tammi M., Virtanen I., Helminen H. J. Influence of short-term hydrostatic pressure on organization of stress fibers in cultured chondrocytes. J Orthop Res. 1995 Jul;13(4):495–502. doi: 10.1002/jor.1100130404. [DOI] [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Donlon B. S., Gupta M. K., Mohtai M., Das P., Carter D. R., Cooke J., Gibbons G., Hutchinson N., Schurman D. J. Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J Orthop Res. 1995 Nov;13(6):824–831. doi: 10.1002/jor.1100130604. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Rusk S. F., Ellison B. E., Wessells P., Tsuchiya K., Carter D. R., Caler W. E., Sandell L. J., Schurman D. J. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996 Jan;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- Sotozono C., Kinoshita S., Kita M., Imanishi J. Paracrine role of keratinocyte growth factor in rabbit corneal epithelial cell growth. Exp Eye Res. 1994 Oct;59(4):385–391. doi: 10.1006/exer.1994.1122. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Kubo T., Arai Y., Imanishi J., Kawata M., Hirasawa Y. Localization of heat shock protein in osteoarthritic cartilage. Scand J Rheumatol. 1997;26(5):368–375. doi: 10.3109/03009749709065701. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Kubo T., Goomer R. S., Amiel D., Kobayashi K., Imanishi J., Teshima R., Hirasawa Y. Analysis of heat shock proteins and cytokines expressed during early stages of osteoarthritis in a mouse model. Osteoarthritis Cartilage. 1997 Sep;5(5):321–329. doi: 10.1016/s1063-4584(97)80036-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Kubo T., Kobayashi K., Imanishi J., Takigawa M., Arai Y., Hirasawa Y. Hydrostatic pressure influences mRNA expression of transforming growth factor-beta 1 and heat shock protein 70 in chondrocyte-like cell line. J Orthop Res. 1997 Jan;15(1):150–158. doi: 10.1002/jor.1100150122. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Tajima K., Pan H. O., Enomoto M., Kinoshita A., Suzuki F., Takano Y., Mori Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989 Jul 15;49(14):3996–4002. [PubMed] [Google Scholar]
- Tuckwell D. S., Ayad S., Grant M. E., Takigawa M., Humphries M. J. Conformation dependence of integrin-type II collagen binding. Inability of collagen peptides to support alpha 2 beta 1 binding, and mediation of adhesion to denatured collagen by a novel alpha 5 beta 1-fibronectin bridge. J Cell Sci. 1994 Apr;107(Pt 4):993–1005. doi: 10.1242/jcs.107.4.993. [DOI] [PubMed] [Google Scholar]
- Urban J. P. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994 Oct;33(10):901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- Venn G., Nietfeld J. J., Duits A. J., Brennan F. M., Arner E., Covington M., Billingham M. E., Hardingham T. E. Elevated synovial fluid levels of interleukin-6 and tumor necrosis factor associated with early experimental canine osteoarthritis. Arthritis Rheum. 1993 Jun;36(6):819–826. doi: 10.1002/art.1780360613. [DOI] [PubMed] [Google Scholar]
- Wang N., Butler J. P., Ingber D. E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993 May 21;260(5111):1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wong M., Wuethrich P., Buschmann M. D., Eggli P., Hunziker E. Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J Orthop Res. 1997 Mar;15(2):189–196. doi: 10.1002/jor.1100150206. [DOI] [PubMed] [Google Scholar]
- Wright M. O., Stockwell R. A., Nuki G. Response of plasma membrane to applied hydrostatic pressure in chondrocytes and fibroblasts. Connect Tissue Res. 1992;28(1-2):49–70. doi: 10.3109/03008209209014227. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kampen G. P., Veldhuijzen J. P., Kuijer R., van de Stadt R. J., Schipper C. A. Cartilage response to mechanical force in high-density chondrocyte cultures. Arthritis Rheum. 1985 Apr;28(4):419–424. doi: 10.1002/art.1780280410. [DOI] [PubMed] [Google Scholar]