Abstract
OBJECTIVES—To determine the ability of lactoferrin in rheumatoid arthritis (RA) synovial fluid to bind "free" iron, and to study the regulatory mechanisms therein that control iron homeostasis. METHODS—"Free" iron was determined by the bleomycin assay and lactoferrin concentrations by enzyme linked immunosorbent assay. The activities of iron regulatory protein (IRP) and NF-κB in synovial fluid cells were assayed by mobility shift assay. RESULTS—30% of synovial fluids contained "free" iron and in these, lactoferrin concentrations were significantly lower than in those with no "free" iron (p<0.01). Addition of exogenous lactoferrin consistently reduced the amount of "free" iron in positive synovial fluids. IRP activity in synovial cells did not correlate with synovial fluid iron concentrations but did correlate with NF-κB activation and with serum C reactive protein. CONCLUSION—Lactoferrin may prevent iron mediated tissue damage in RA by reducing "free" synovial iron concentration when inflammatory stimuli have disregulated IRP mediated iron homeostasis. Keywords: lactoferrin; rheumatoid arthritis; inflammation
Full Text
The Full Text of this article is available as a PDF (163.6 KB).
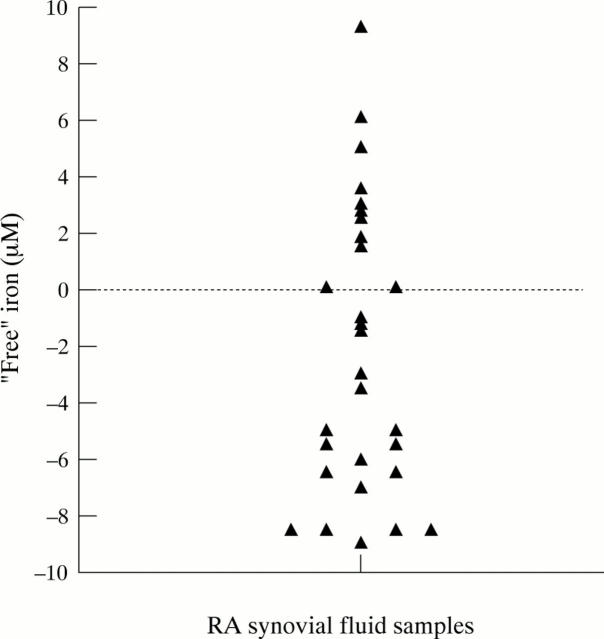
Figure 1 .
"Free" iron in synovial fluid of RA patients. The results represent the concentrations of "free" iron in RA patients using the bleomycin assay at pH 5.3.
Figure 2 .
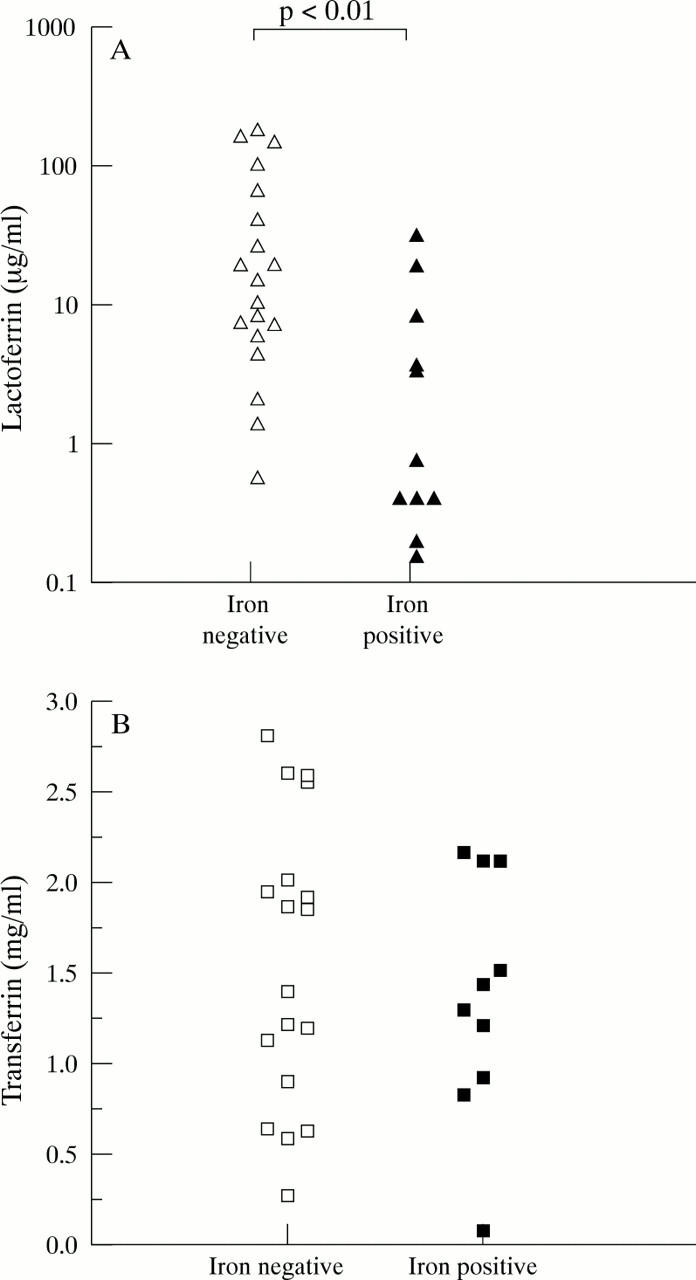
Lactoferrin (A) and transferrin (B) in synovial fluid from RA patients. The results correspond to the synovial fluid lactoferrin and transferrin concentrations in RA patients segregated for the presence or absence of "free" iron. Lactoferrin concentrations were significantly lower (p<0.01) in fluids containing "free" iron. There was no significant difference in transferrin concentrations.
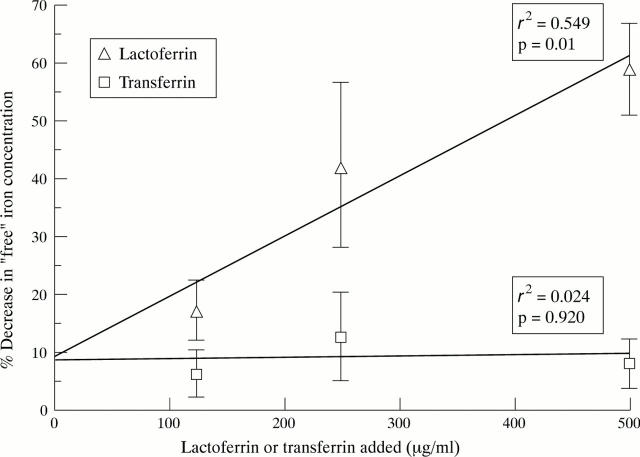
Figure 3 .
Binding of iron in synovial fluid by exogenous lactoferrin and transferrin. Synovial fluid samples were incubated overnight at 4°C with different concentrations of lactoferrin or transferrin and "free" iron was measured by the bleomycin assay. The results show the percentage decrease in "free" iron (mean (SEM); n=7) for each concentration of exogenous lactoferrin or transferrin.
Figure 4 .
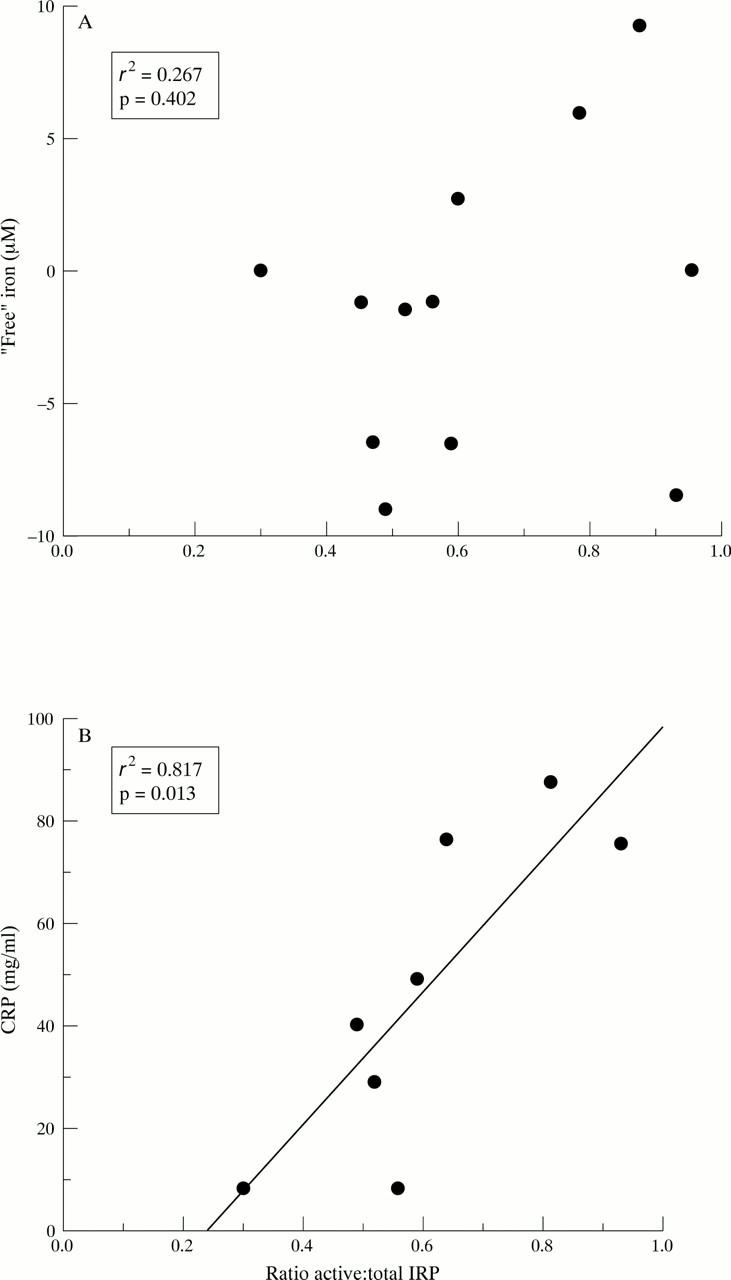
Correlation between iron regulatory protein (IRP) activity in synovial fluid cells of RA patients with "free" iron (A) and CRP (B). IRP activity was calculated from the ratio of intensity of IRE-IRP complexes in mobility shift assays performed with or without 2-mercaptoethanol. Serum CRP concentrations correlated with IRP activity (p=0.013)
Figure 5 .
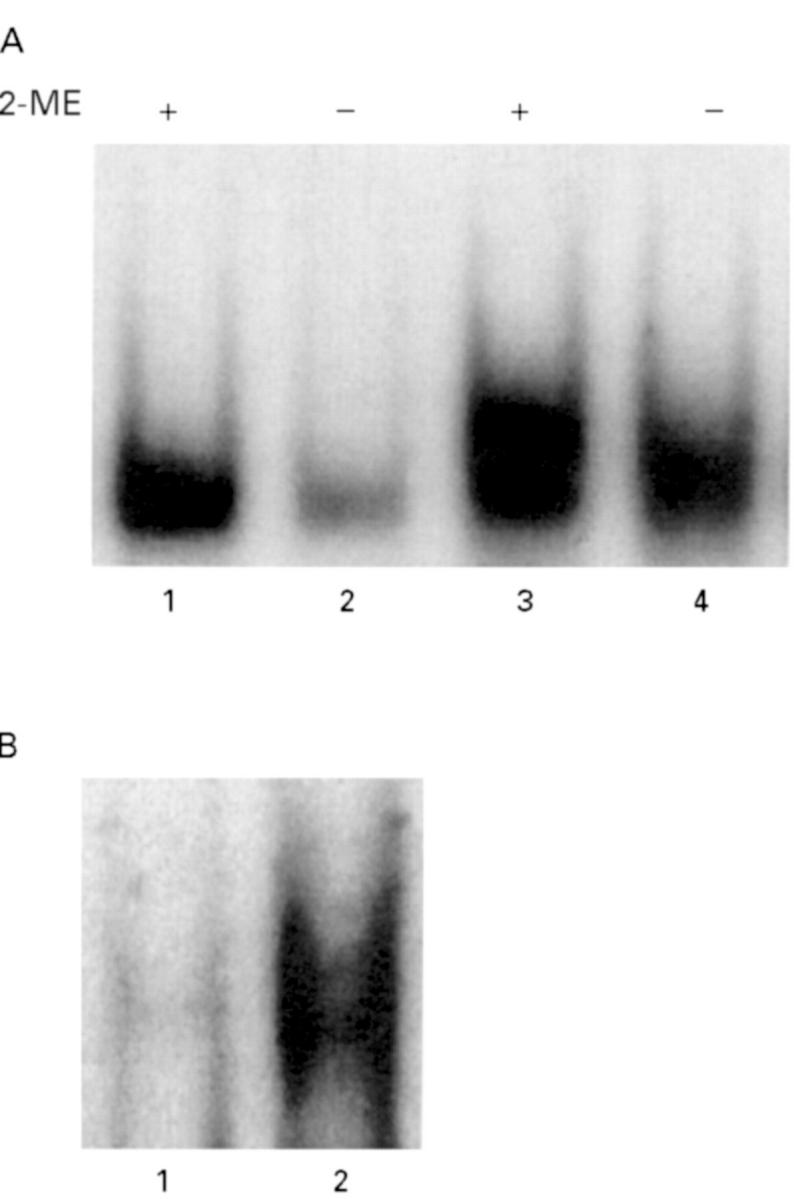
Mobility shift assay of IRP (A) and NFκB (B) from synovial fluid mononuclear and polymorphonuclear cells from a representative case of three RA patients. In panel A, lanes 1 and 2 correspond to active IRP in synovial fluid mononuclear cells and lanes 3 and 4 to active IRP in synovial fluid polymorphonuclear cells. Samples in lanes 1 and 3 were treated with 2-mercaptoethanol (2-ME) to convert inactive IRP to the active form (see methods). In panel B, lane 1 corresponds to NFκB activity in synovial fluid mononuclear cells and lane 2 to NFκB activity in synovial fluid polymorphonuclear cells.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afeltra A., Sebastiani G. D., Galeazzi M., Caccavo D., Ferri G. M., Marcolongo R., Bonomo L. Antineutrophil cytoplasmic antibodies in synovial fluid and in serum of patients with rheumatoid arthritis and other types of synovitis. J Rheumatol. 1996 Jan;23(1):10–15. [PubMed] [Google Scholar]
- Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972 Feb 29;257(2):314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Andrews F. J., Morris C. J., Kondratowicz G., Blake D. R. Effect of iron chelation on inflammatory joint disease. Ann Rheum Dis. 1987 Apr;46(4):327–333. doi: 10.1136/ard.46.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Gallagher P. J., Potter A. R., Bell M. J., Bacon P. A. The effect of synovial iron on the progression of rheumatoid disease. A histologic assessment of patients with early rheumatoid synovitis. Arthritis Rheum. 1984 May;27(5):495–501. doi: 10.1002/art.1780270503. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Lunec J., Williams A., Good P. A., Crewes S. J., Gutteridge J. M., Rowley D., Halliwell B., Cornish A. Cerebral and ocular toxicity induced by desferrioxamine. Q J Med. 1985 Jul;56(219):345–355. [PubMed] [Google Scholar]
- Brock J. H., Lamont A., Boyle D. J., Holme E. R., McSharry C., Bunn J. E., Lönnerdal B. Antibodies to lactoferrin--a possible link between cow's milk intolerance and autoimmune disease. Biochem Soc Trans. 1997 May;25(2):317S–317S. doi: 10.1042/bst025317s. [DOI] [PubMed] [Google Scholar]
- Burkhardt H., Schwingel M., Menninger H., Macartney H. W., Tschesche H. Oxygen radicals as effectors of cartilage destruction. Direct degradative effect on matrix components and indirect action via activation of latent collagenase from polymorphonuclear leukocytes. Arthritis Rheum. 1986 Mar;29(3):379–387. doi: 10.1002/art.1780290311. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Levinson J. E., Bass J. C., Baum J., Brewer E. J., Jr, Fink C. W., Hanson V., Jacobs J. C., Masi A. T., Schaller J. G. A study of classification criteria for a diagnosis of juvenile rheumatoid arthritis. Arthritis Rheum. 1986 Feb;29(2):274–281. doi: 10.1002/art.1780290216. [DOI] [PubMed] [Google Scholar]
- Espinoza L. R., Cuéllar M. L., Silveira L. H. Psoriatic arthritis. Curr Opin Rheumatol. 1992 Aug;4(4):470–478. [PubMed] [Google Scholar]
- Etherington D. J., Pugh D., Silver I. A. Collagen degradation in an experimental inflammatory lesion: studies on the role of the macrophage. Acta Biol Med Ger. 1981;40(10-11):1625–1636. [PubMed] [Google Scholar]
- Evans P. J., Halliwell B. Measurement of iron and copper in biological systems: bleomycin and copper-phenanthroline assays. Methods Enzymol. 1994;233:82–92. doi: 10.1016/s0076-6879(94)33010-7. [DOI] [PubMed] [Google Scholar]
- Gray N. K., Quick S., Goossen B., Constable A., Hirling H., Kühn L. C., Hentze M. W. Recombinant iron-regulatory factor functions as an iron-responsive-element-binding protein, a translational repressor and an aconitase. A functional assay for translational repression and direct demonstration of the iron switch. Eur J Biochem. 1993 Dec 1;218(2):657–667. doi: 10.1111/j.1432-1033.1993.tb18420.x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Bleomycin-detectable iron in knee-joint synovial fluid from arthritic patients and its relationship to the extracellular antioxidant activities of caeruloplasmin, transferrin and lactoferrin. Biochem J. 1987 Jul 15;245(2):415–421. doi: 10.1042/bj2450415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hershko C., Graham G., Bates G. W., Rachmilewitz E. A. Non-specific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978 Oct;40(2):255–263. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Lestas A. N. The effect of pH upon human transferrin: selective labelling of the two iron-binding sites. Br J Haematol. 1976 Mar;32(3):341–350. doi: 10.1111/j.1365-2141.1976.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes I. B., Leung B. P., Field M., Wei X. Q., Huang F. P., Sturrock R. D., Kinninmonth A., Weidner J., Mumford R., Liew F. Y. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996 Oct 1;184(4):1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melefors O., Hentze M. W. Iron regulatory factor--the conductor of cellular iron regulation. Blood Rev. 1993 Dec;7(4):251–258. doi: 10.1016/0268-960x(93)90012-s. [DOI] [PubMed] [Google Scholar]
- Pantopoulos K., Weiss G., Hentze M. W. Nitric oxide and oxidative stress (H2O2) control mammalian iron metabolism by different pathways. Mol Cell Biol. 1996 Jul;16(7):3781–3788. doi: 10.1128/mcb.16.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez L., Calvo M., Brock J. H. Biological role of lactoferrin. Arch Dis Child. 1992 May;67(5):657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]