Abstract
OBJECTIVE—The objective of this study was to detail the topographical and zonal distribution of α and β subunits of the integrin superfamily in normal and osteoarthritic cartilage. METHODS—Immunohistochemistry utilising antibodies towards α and β subunits was performed on cryostat sections of human articular cartilage from macroscopically normal (n = 6) and osteoarthritic (n = 6) femoral heads. Samples of articular cartilage were obtained from 12 topographically distinct sites from each femoral head. Each section was divided into zones (superficial, middle, deep) and staining scores were recorded. RESULTS—Normal cartilage stained for integrin subunits α1, α5, αV, β1, β4, and β5, but not for α2, α3, α4, α6, β2, β3, and β6. Intact and non-intact residual cartilage from osteoarthritic femoral heads stained for α1, α2, α5, αV, β1, β4, and β5. Staining was occasionally seen for α4 and β2, but not for α3, α6, β3, and β6. There was no topographical variation in the staining for any of the subunits in either normal or osteoarthritic cartilage. The only subunit that displayed a zonal variation was αV; staining for this subunit was most pronounced in the superficial zone compared with the middle and deep zones. CONCLUSION—Chondrocytes in normal and osteoarthritic cartilage express the integrin subunits α1, α5, αV, β1, β4, and β5. Chondrocytes in osteoarthritic cartilage, in addition, express the α2, α4, and β2 subunits. The αv subunit is expressed by more chondrocytes in the superficial zone in comparison with cells in the deeper zones. None of the subunits display topographical variation in expression. Keywords: cartilage; integrins; immunohistochemistry; osteoarthritis
Full Text
The Full Text of this article is available as a PDF (145.4 KB).
Figure 1 .
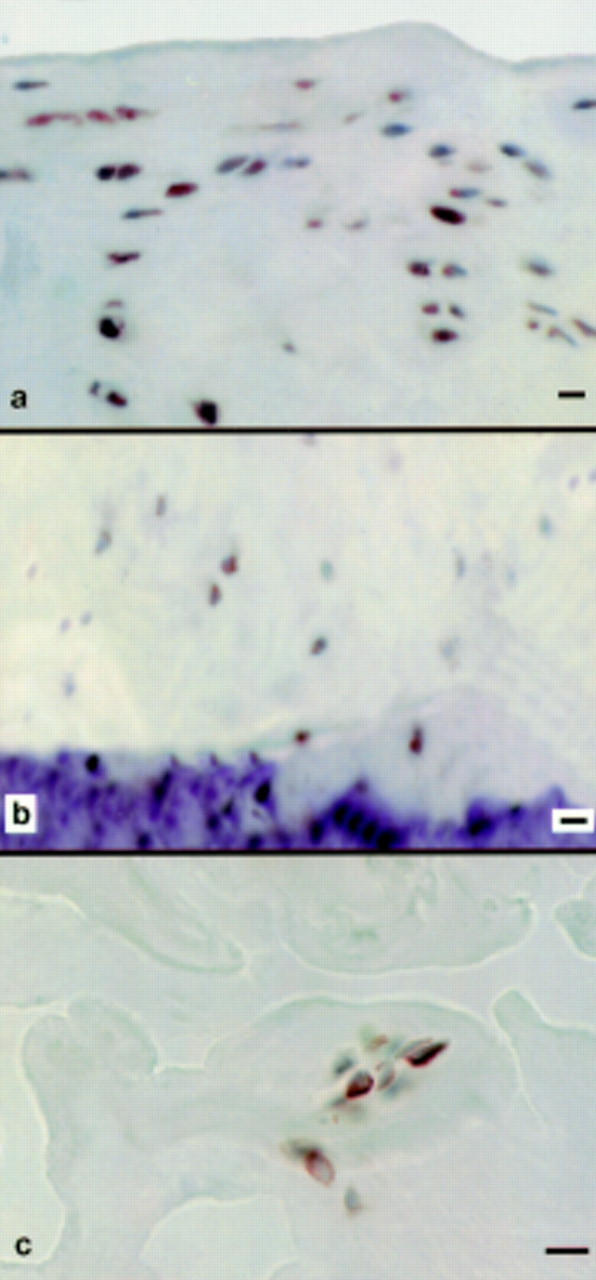
Articular cartilage stained for the αV integrin subunit. (a) The majority of chondrocytes are αV positive in the superficial zone of articular cartilage from a macroscopically normal femoral head. (b) Both αV positive and αV negative chondrocytes in the deep zone of articular cartilage from a macroscopically normal femoral head. (c) A cluster containing both αV positive and αV negative chondrocytes in non-intact articular cartilage from an osteoarthritic femoral head; the cartilage contains several clefts. Immunohistochemistry with an immunoperoxidase technique and haematoxylin counterstaining was used (bar marker: 25 µm).
Figure 2 .
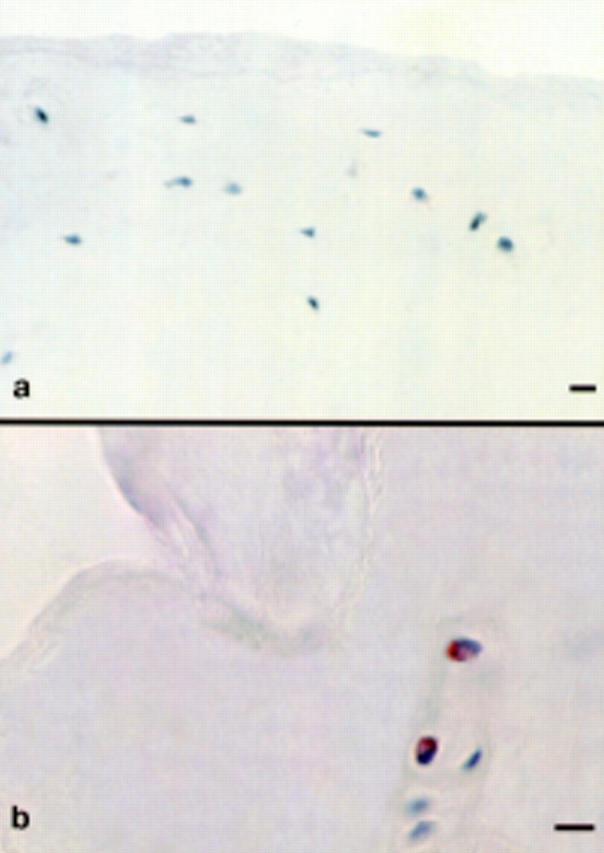
Articular cartilage stained for the α2 integrin subunit. (a) α2 negative chondrocytes in the superficial zone of articular cartilage from a macroscopically normal femoral head. (b) A cluster containing both α2 positive and α2 negative chondrocytes in non-intact articular cartilage from an osteoarthritic femoral head. Immunohistochemistry with an immunoperoxidase technique and haematoxylin counterstaining was used (bar marker: 25 µm).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Deyl Z. Altered expression of collagen phenotype in osteoarthrosis. Clin Chim Acta. 1983 Sep 15;133(1):25–32. doi: 10.1016/0009-8981(83)90017-7. [DOI] [PubMed] [Google Scholar]
- Aigner T., Dietz U., Stöss H., von der Mark K. Differential expression of collagen types I, II, III, and X in human osteophytes. Lab Invest. 1995 Aug;73(2):236–243. [PubMed] [Google Scholar]
- Armstrong C. G., Gardner D. L. Thickness and distribution of human femoral head articular cartilage. Changes with age. Ann Rheum Dis. 1977 Oct;36(5):407–412. doi: 10.1136/ard.36.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner E. C., Tortorella M. D. Signal transduction through chondrocyte integrin receptors induces matrix metalloproteinase synthesis and synergizes with interleukin-1. Arthritis Rheum. 1995 Sep;38(9):1304–1314. doi: 10.1002/art.1780380919. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Davidson C., Woodhouse S. M., Osborne D. J. Chondroitin sulphation in human joint tissues varies with age, zone and topography. Acta Orthop Scand Suppl. 1995 Oct;266:22–25. [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Shaffer J. D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982 Aug;30(1):215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Bulstra S. K., Buurman W. A., Walenkamp G. H., Van der Linden A. J. Metabolic characteristics of in vitro cultured human chondrocytes in relation to the histopathologic grade of osteoarthritis. Clin Orthop Relat Res. 1989 May;(242):294–302. [PubMed] [Google Scholar]
- COLLINS D. H., McELLIGOTT T. F. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteoarthritic human articular cartilage. Ann Rheum Dis. 1960 Dec;19:318–330. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. E., Dingle J. T., Pigott R., Power C., Sharma H. Expression of intercellular adhesion molecule 1 (ICAM-1) on human articular cartilage chondrocytes. Connect Tissue Res. 1991;26(3):207–216. doi: 10.3109/03008209109152439. [DOI] [PubMed] [Google Scholar]
- Dürr J., Goodman S., Potocnik A., von der Mark H., von der Mark K. Localization of beta 1-integrins in human cartilage and their role in chondrocyte adhesion to collagen and fibronectin. Exp Cell Res. 1993 Aug;207(2):235–244. doi: 10.1006/excr.1993.1189. [DOI] [PubMed] [Google Scholar]
- Gay S., Müller P. K., Lemmen C., Remberger K., Matzen K., Kühn K. Immunohistological study on collagen in cartilage-bone metamorphosis and degenerative osteoarthrosis. Klin Wochenschr. 1976 Oct 15;54(20):969–976. doi: 10.1007/BF01468947. [DOI] [PubMed] [Google Scholar]
- Hamerman D. The biology of osteoarthritis. N Engl J Med. 1989 May 18;320(20):1322–1330. doi: 10.1056/NEJM198905183202006. [DOI] [PubMed] [Google Scholar]
- Huang D. Extracellular matrix-cell interactions and chondrogenesis. Clin Orthop Relat Res. 1977 Mar-Apr;(123):169–176. [PubMed] [Google Scholar]
- Hughes D. E., Salter D. M., Dedhar S., Simpson R. Integrin expression in human bone. J Bone Miner Res. 1993 May;8(5):527–533. doi: 10.1002/jbmr.5650080503. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jobanputra P., Corrigall V., Kingsley G., Panayi G. Cellular responses to human chondrocytes: absence of allogeneic responses in the presence of HLA-DR and ICAM-1. Clin Exp Immunol. 1992 Nov;90(2):336–344. doi: 10.1111/j.1365-2249.1992.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobanputra P., Lin H., Jenkins K., Bavington C., Brennan F. R., Nuki G., Salter D. M., Godolphin J. L. Modulation of human chondrocyte integrins by inflammatory synovial fluid. Arthritis Rheum. 1996 Aug;39(8):1430–1432. doi: 10.1002/art.1780390826. [DOI] [PubMed] [Google Scholar]
- Jones K. L., Brown M., Ali S. Y., Brown R. A. An immunohistochemical study of fibronectin in human osteoarthritic and disease free articular cartilage. Ann Rheum Dis. 1987 Nov;46(11):809–815. doi: 10.1136/ard.46.11.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. K., Moran M. E., Salter R. B. The potential for regeneration of articular cartilage in defects created by chondral shaving and subchondral abrasion. An experimental investigation in rabbits. J Bone Joint Surg Am. 1991 Oct;73(9):1301–1315. [PubMed] [Google Scholar]
- Lapadula G., Iannone F., Zuccaro C., Grattagliano V., Covelli M., Patella V., Lo Bianco G., Pipitone V. Integrin expression on chondrocytes: correlations with the degree of cartilage damage in human osteoarthritis. Clin Exp Rheumatol. 1997 May-Jun;15(3):247–254. [PubMed] [Google Scholar]
- Lee G. M., Johnstone B., Jacobson K., Caterson B. The dynamic structure of the pericellular matrix on living cells. J Cell Biol. 1993 Dec;123(6 Pt 2):1899–1907. doi: 10.1083/jcb.123.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheum. 1993 Aug;36(8):1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Maroudas A., Venn M. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. II. Swelling. Ann Rheum Dis. 1977 Oct;36(5):399–406. doi: 10.1136/ard.36.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. R., Mankin H. J., Shoji H., D'Ambrosia R. D. Identification of fibronectin in preparations of osteoarthritic human cartilage. Connect Tissue Res. 1984;12(3-4):267–275. doi: 10.3109/03008208409013689. [DOI] [PubMed] [Google Scholar]
- Mitrovic D., Quintero M., Stankovic A., Ryckewaert A. Cell density of adult human femoral condylar articular cartilage. Joints with normal and fibrillated surfaces. Lab Invest. 1983 Sep;49(3):309–316. [PubMed] [Google Scholar]
- Németh-Csóka M., Mészáros T. Minor collagens in arthrotic human cartilage. Change in content of 1 alpha, 2 alpha, 3 alpha and M-collagen with age and in osteoarthrosis. Acta Orthop Scand. 1983 Aug;54(4):613–619. doi: 10.3109/17453678308992898. [DOI] [PubMed] [Google Scholar]
- Ostergaard K., Petersen J., Andersen C. B., Bendtzen K., Salter D. M. Histologic/histochemical grading system for osteoarthritic articular cartilage: reproducibility and validity. Arthritis Rheum. 1997 Oct;40(10):1766–1771. doi: 10.1002/art.1780401007. [DOI] [PubMed] [Google Scholar]
- Ostergaard K., Salter D. M., Andersen C. B., Petersen J., Bendtzen K. CD44 expression is up-regulated in the deep zone of osteoarthritic cartilage from human femoral heads. Histopathology. 1997 Nov;31(5):451–459. doi: 10.1046/j.1365-2559.1997.2760879.x. [DOI] [PubMed] [Google Scholar]
- Ronzière M. C., Ricard-Blum S., Tiollier J., Hartmann D. J., Garrone R., Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990 Apr 19;1038(2):222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. M., Godolphin J. L., Gourlay M. S. Chondrocyte heterogeneity: immunohistologically defined variation of integrin expression at different sites in human fetal knees. J Histochem Cytochem. 1995 Apr;43(4):447–457. doi: 10.1177/43.4.7897185. [DOI] [PubMed] [Google Scholar]
- Salter D. M., Godolphin J. L., Gourlay M. S., Lawson M. F., Hughes D. E., Dunne E. Analysis of human articular chondrocyte CD44 isoform expression and function in health and disease. J Pathol. 1996 Aug;179(4):396–402. doi: 10.1002/(SICI)1096-9896(199608)179:4<396::AID-PATH606>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Salter D. M., Hughes D. E., Simpson R., Gardner D. L. Integrin expression by human articular chondrocytes. Br J Rheumatol. 1992 Apr;31(4):231–234. doi: 10.1093/rheumatology/31.4.231. [DOI] [PubMed] [Google Scholar]
- Venn M., Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977 Apr;36(2):121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. D., Fischer M., Gannon J., Thompson R. C., Jr, Oegema T. R., Jr Expression of type-X collagen in osteoarthritis. J Orthop Res. 1995 Jan;13(1):4–12. doi: 10.1002/jor.1100130104. [DOI] [PubMed] [Google Scholar]
- Woods V. L., Jr, Schreck P. J., Gesink D. S., Pacheco H. O., Amiel D., Akeson W. H., Lotz M. Integrin expression by human articular chondrocytes. Arthritis Rheum. 1994 Apr;37(4):537–544. doi: 10.1002/art.1780370414. [DOI] [PubMed] [Google Scholar]
- Yonezawa I., Kato K., Yagita H., Yamauchi Y., Okumura K. VLA-5-mediated interaction with fibronectin induces cytokine production by human chondrocytes. Biochem Biophys Res Commun. 1996 Feb 6;219(1):261–265. doi: 10.1006/bbrc.1996.0215. [DOI] [PubMed] [Google Scholar]
- von der Mark K., Kirsch T., Nerlich A., Kuss A., Weseloh G., Glückert K., Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992 Jul;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]


