Abstract
OBJECTIVE—Methotrexate (MTX) has become the disease modifying drug of choice for the treatment of rheumatoid arthritis (RA). Direct effects of MTX on articular cartilage in vivo and in vitro were studied to determine possible adverse effects of the drug. METHODS—For in vitro experiments, adult bovine articular cartilage explants were cultured in the presence of MTX (0 to 100 µM), and effects on DNA and matrix metabolism were studied. For in vivo studies, 48 adult female rabbits were treated with MTX (30 mg/kg/week intramuscularly) or placebo, respectively, for up to 12 weeks, and effects on the cartilage of the femoral condyles were assessed. RESULTS—In vitro, MTX dose dependently increased the uptake of [3H]-thymidine, and decreased incorporation of [3H]-d-uridine into chondrocytes with a half maximal effect at 0.03 µM, suggesting inhibition of thymidylate-synthetase activity by the drug. MTX also dose dependently reduced the proportion of chondrocytes in S-phase, as determined by flow cytometry. MTX did not affect LDH release from chondrocytes or the proportion of viable cells, nor did it change the rate of protein synthesis, proteoglycan synthesis, proteoglycan breakdown, or the hydrodynamic size of newly synthesised proteoglycans. In vivo, MTX did not appreciably affect proteoglycan synthesis of the chondrocytes, proteoglycan content of the cartilage matrix, density of the chondrocyte population, or histological integrity of the cartilage. CONCLUSIONS—The data suggest the absence of major adverse effects by MTX on articular cartilage proteoglycan metabolism. Chondrocyte DNA metabolism seems to be changed by MTX only in concentrations and exposition periods clearly exceeding those found in synovial fluid of RA patients receiving the commonly prescribed doses of the drug. Keywords: methotrexate; articular cartilage; rheumatoid arthritis
Full Text
The Full Text of this article is available as a PDF (138.2 KB).
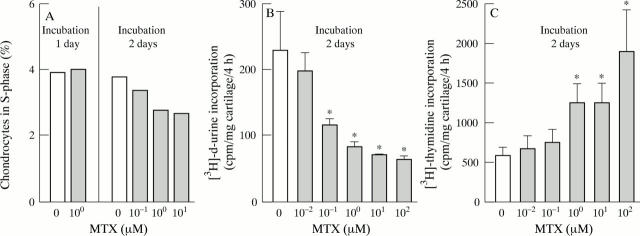
Figure 1 .
Effects of MTX on the DNA metabolism of cultured articular cartilage. (A) Reduction of the proportion of chondrocytes in S-phase by MTX in vitro. Bovine articular cartilage explants were cultured in the presence of various MTX concentrations, then isolated with collagenase, stained with propidium iodide, and analysed for DNA content by flow cytometry. The proportion of chondrocytes in S-phase was dose dependently reduced by MTX after 48 hours of culture (p<0.05; linear regression analysis), but not after 24 hours. Bars represent means of at least 10 000 events. (B) Inhibition of [3H]-d-uridine-incorporation into chondrocytes by MTX in vitro. Bovine articular cartilage explants were cultured in the presence of various MTX concentrations for two days, then labelled with [3H]-d-uridine for four hours, washed in PBS to remove free [3H]-d-uridine, digested with papain, and counted for [3H]. Bars represent means and SEM of eight cartilage samples per group; * p < 0.05 v control without MTX (t test). (C) Stimulation of [3H]-thymidine incorporation into chondrocytes by MTX in vitro. Bovine articular cartilage explants were cultured in the presence of various MTX concentrations for two days, then labelled with [3H]-thymidine for four hours, washed in PBS to remove free [3H]-thymidine, digested with papain, and counted for [3H]. Bars represent means and SEM of eight cartilage samples per group; * p<0.05 v control without MTX (t test).
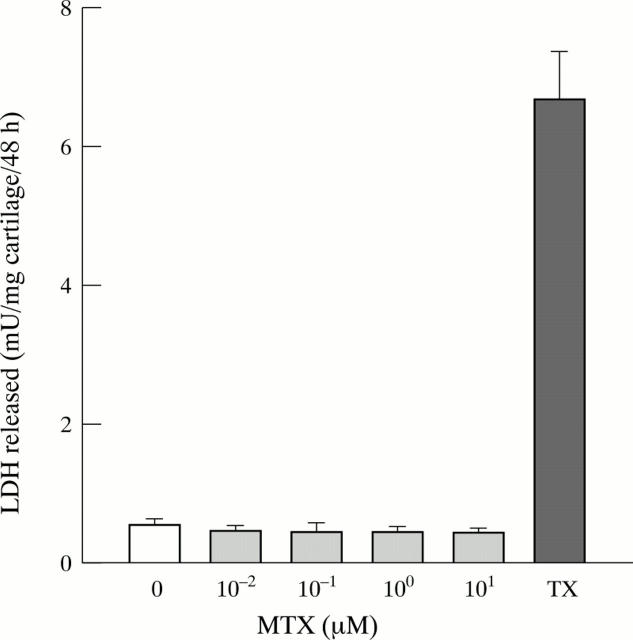
Figure 2 .
No effect of MTX on LDH release from cultured cartilage. Bovine articular cartilage was incubated for two days in the presence of MTX (0 to 10 µM), and the amount of lactate dehydrogenase (LDH) released into the culture medium determined fluorometrically. The detergent Triton-X 100 (TX) was used as a positive control. Bars represent means and SEM of eight cartilage explants per group.
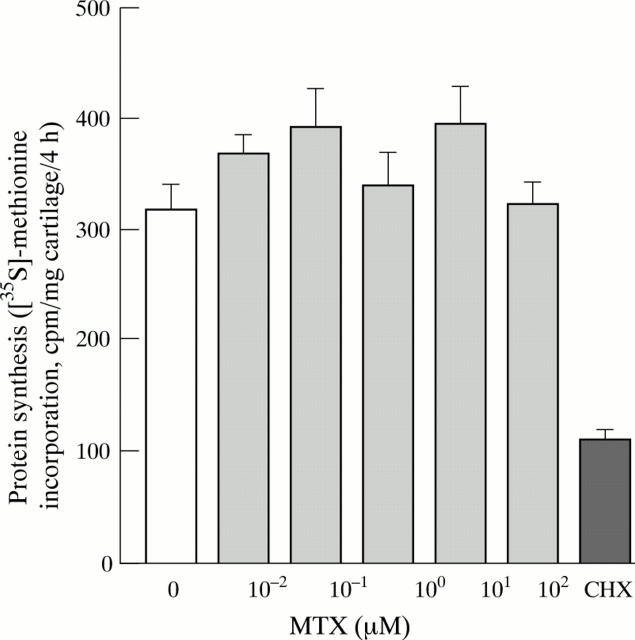
Figure 3 .
No inhibition by MTX of chondrocyte protein synthesis in vitro. Bovine articular cartilage was incubated for two days in the presence of MTX (0 to 100 µM), or cycloheximide (CHX) (10 mg/l). The samples were subsequently labelled with [35S]-methionine for four hours, hydrolysed with papain, and incorporation of the tracer into trichloroacetic acid precipitable macromolecules was determined. Bars represent means and SEM of eight cartilage explants per group.
Figure 4 .
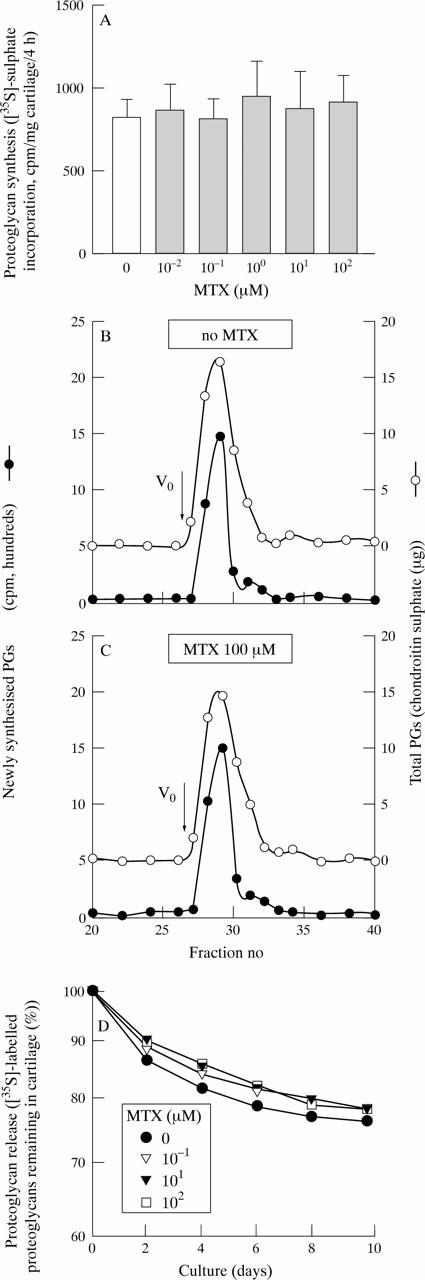
No effects of MTX on cartilage proteoglycan metabolism in culture. (A) No inhibition by MTX of chondrocyte proteoglycan synthesis in vitro. Bovine articular cartilage was incubated for two days in the presence of MTX (0 to 100 µM). The samples were subsequently labelled with [35S]-sulphate for four hours, hydrolysed with papain, and incorporation of the tracer into cetyl-pyridiumchloride precipitable macromolecules was determined. Bars represent means and SEM of eight cartilage explants per group. Identical results were obtained, when cartilage was cultured for 4, 8, or 16 days in the presence of MTX (not shown). (B) and (C) No alteration by MTX of the hydrodynamic size of newly synthesised (solid circles) or total (open circles) proteoglycan monomers in cultured articular cartilage. Bovine cartilage explants were incubated for 16 days in the absence (B) or presence (100 µM) of MTX (C). Media were replaced every two days, keeping the respective MTX concentrations (that is, 0 or 100 µM) constant throughout the entire incubation period. The samples were then labelled with [35S]-sulphate for four hours by addition of the tracer. The PGs were extracted from the cartilage using 4 M guanidine-HCl, and subjected to size exclusion chromatography on a Sephacryl-S-200-HR column under dissociative conditions. In the respective fractions, the newly synthesised PGs (as [35S]; solid circles) were determined by liquid scintillation counting, and the total PGs (as chondroitin sulphate (CS); open circles) measured using a dimethyl-methyleneblue dye binding assay. The smooth lines are based on a peak analysis performed with the SIGMA-Plot software package for microcomputers (Jandel Scientific, Erkrath, Germany). The arrows indicate v0 of the column, vt corresponds to fraction no 77. (D) No MTX effect on the spontaneous PG release from articular cartilage in culture. Bovine articular cartilage explants were labelled with [35S]-sulphate for four hours. Free sulphate was subsequently removed by repeatedly washing the samples in PBS (verified by size exclusion chromatography). Incubation was then continued for 10 days in the presence of various MTX concentrations. Media were harvested every two days and replaced with fresh media containing MTX in concentrations corresponding to the respective treatment group. Radiolabelled PGs contained in the harvested media, and PGs remaining in the cartilage samples, respectively, were determined by liquid scintillation counting as described in methods. The rate of PG release was then calculated.
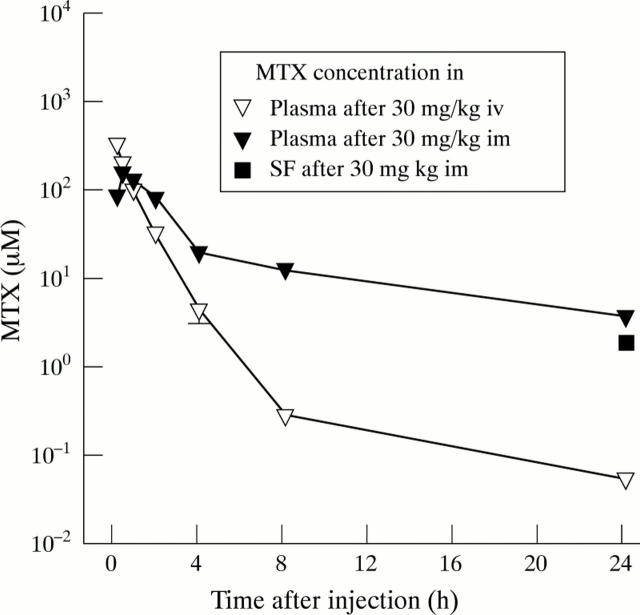
Figure 5 .
Pharmacokinetics of MTX in adult rabbits. MTX (30 mg/kg body weight) was administered intravenously (n=3), or intramuscularly (n=5). Serial plasma samples were then obtained, and MTX concentrations measured fluorometrically. The MTX concentrations in the synovial fluid (SF) of the knee joints of the animals were determined 24 hours after intramuscular injection of 30 mg MTX/kg body weight (n=5), as described in methods. Symbols represent means and SEM of the indicated number of animals. Most error bars do not exceed the size of their respective symbol.
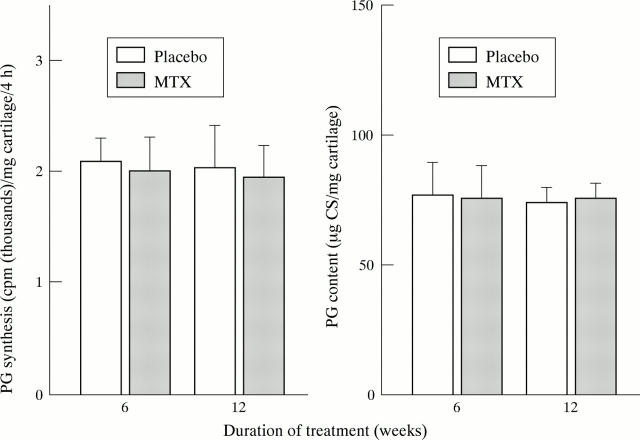
Figure 6 .
No significant effects of systemic MTX on PG synthesis or PG content of articular cartilage in adult rabbits. Rabbits were treated with MTX (30 mg/week/kg intramuscularly) or placebo, respectively, for 6 or 12 weeks, and effects on the PG metabolism of the cartilage of the femoral condyles were assessed as described in methods. Bars represent means and SEM of eight animals per group (treatment for six weeks), or 16 animals per group (treatment for 12 weeks). CS = chondroitin sulphate.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asako H., Kubes P., Baethge B. A., Wolf R. E., Granger D. N. Colchicine and methotrexate reduce leukocyte adherence and emigration in rat mesenteric venules. Inflammation. 1992 Feb;16(1):45–56. doi: 10.1007/BF00917514. [DOI] [PubMed] [Google Scholar]
- Crossley M. J., Spowage M., Hunneyball I. M. Studies on the effects of pharmacological agents on antigen-induced arthritis in BALB/c mice. Drugs Exp Clin Res. 1987;13(5):273–277. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Boyle D. L. Mechanisms of methotrexate action in rheumatoid arthritis. Selective decrease in synovial collagenase gene expression. Arthritis Rheum. 1994 Feb;37(2):193–200. doi: 10.1002/art.1780370207. [DOI] [PubMed] [Google Scholar]
- Foong W. C., Green K. L. Treatment of antigen-induced arthritis in rabbits with liposome-entrapped methotrexate injected intra-articularly. J Pharm Pharmacol. 1993 Mar;45(3):204–209. doi: 10.1111/j.2042-7158.1993.tb05533.x. [DOI] [PubMed] [Google Scholar]
- Fries J. F., Williams C. A., Morfeld D., Singh G., Sibley J. Reduction in long-term disability in patients with rheumatoid arthritis by disease-modifying antirheumatic drug-based treatment strategies. Arthritis Rheum. 1996 Apr;39(4):616–622. doi: 10.1002/art.1780390412. [DOI] [PubMed] [Google Scholar]
- Hunneyball I. M., Crossley M. J., Spowage M. Pharmacological studies of antigen-induced arthritis in BALB/c mice. II. The effects of second-line antirheumatic drugs and cytotoxic agents on the histopathological changes. Agents Actions. 1986 Jun;18(3-4):394–400. doi: 10.1007/BF01965003. [DOI] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Mannoni A., Altman R. D., Muniz O. E., Serni U., Dean D. D. The effects of methotrexate on normal and osteoarthritic lapine articular cartilage. J Rheumatol. 1993 May;20(5):849–855. [PubMed] [Google Scholar]
- Martel-Pelletier J., Cloutier J. M., Pelletier J. P. In vivo effects of antirheumatic drugs on neutral collagenolytic proteases in human rheumatoid arthritis cartilage and synovium. J Rheumatol. 1988 Aug;15(8):1198–1204. [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P. Degradative changes in human articular cartilage induced by chemotherapeutic agents. J Rheumatol. 1986 Feb;13(1):164–174. [PubMed] [Google Scholar]
- McQuillan D. J., Handley C. J., Robinson H. C., Ng K., Tzaicos C., Brooks P. R., Lowther D. A. The relation of protein synthesis to chondroitin sulphate biosynthesis in cultured bovine cartilage. Biochem J. 1984 Dec 15;224(3):977–988. doi: 10.1042/bj2240977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidel J., Zeidler U. Independent effects of interleukin 1 on proteoglycan synthesis and proteoglycan breakdown of bovine articular cartilage in vitro. Agents Actions. 1993 May;39(1-2):82–90. doi: 10.1007/BF01975718. [DOI] [PubMed] [Google Scholar]
- Segal R., Mozes E., Yaron M., Tartakovsky B. The effects of methotrexate on the production and activity of interleukin-1. Arthritis Rheum. 1989 Apr;32(4):370–377. doi: 10.1002/anr.1780320403. [DOI] [PubMed] [Google Scholar]
- Thomas R., Carroll G. J. Reduction of leukocyte and interleukin-1 beta concentrations in the synovial fluid of rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 1993 Sep;36(9):1244–1252. doi: 10.1002/art.1780360909. [DOI] [PubMed] [Google Scholar]
- Tishler M., Caspi D., Graff E., Segal R., Peretz H., Yaron M. Synovial and serum levels of methotrexate during methotrexate therapy of rheumatoid arthritis. Br J Rheumatol. 1989 Oct;28(5):422–423. doi: 10.1093/rheumatology/28.5.422. [DOI] [PubMed] [Google Scholar]
- Treadwell B. V., Neidel J., Pavia M., Towle C. A., Trice M. E., Mankin H. J. Purification and characterization of collagenase activator protein synthesized by articular cartilage. Arch Biochem Biophys. 1986 Dec;251(2):715–723. doi: 10.1016/0003-9861(86)90381-4. [DOI] [PubMed] [Google Scholar]
- Treadwell B. V., Towle C. A., Ishizue K., Mankin K. P., Pavia M., Ollivierre F. M., Gray D. H. Stimulation of the synthesis of collagenase activator protein in cartilage by a factor present in synovial-conditioned medium. Arch Biochem Biophys. 1986 Dec;251(2):724–731. doi: 10.1016/0003-9861(86)90382-6. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Insulin-like growth factor 1 can decrease degradation and promote synthesis of proteoglycan in cartilage exposed to cytokines. Biochem J. 1989 Jun 1;260(2):543–548. doi: 10.1042/bj2600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinblatt M. E., Coblyn J. S., Fox D. A., Fraser P. A., Holdsworth D. E., Glass D. N., Trentham D. E. Efficacy of low-dose methotrexate in rheumatoid arthritis. N Engl J Med. 1985 Mar 28;312(13):818–822. doi: 10.1056/NEJM198503283121303. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Kaplan H., Germain B. F., Block S., Solomon S. D., Merriman R. C., Wolfe F., Wall B., Anderson L., Gall E. Methotrexate in rheumatoid arthritis. A five-year prospective multicenter study. Arthritis Rheum. 1994 Oct;37(10):1492–1498. doi: 10.1002/art.1780371013. [DOI] [PubMed] [Google Scholar]
- Weinblatt M. E., Weissman B. N., Holdsworth D. E., Fraser P. A., Maier A. L., Falchuk K. R., Coblyn J. S. Long-term prospective study of methotrexate in the treatment of rheumatoid arthritis. 84-month update. Arthritis Rheum. 1992 Feb;35(2):129–137. doi: 10.1002/art.1780350202. [DOI] [PubMed] [Google Scholar]
- Willkens R. F. Resolve: methotrexate is the drug of choice after NSAIDs in rheumatoid arthritis. Semin Arthritis Rheum. 1990 Oct;20(2):76–80. doi: 10.1016/0049-0172(90)90020-g. [DOI] [PubMed] [Google Scholar]
- van de Putte L. B., Boerbooms A. M., Barrera P., Kerstens P. J., Jeurissen M. E. Methotrexate: anti-inflammatory or immunosuppressive? Clin Exp Rheumatol. 1993 Mar-Apr;11 (Suppl 8):S97–S99. [PubMed] [Google Scholar]
- van der Sluijs J. A., Geesink R. G., van der Linden A. J., Bulstra S. K., Kuyer R., Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992 Jan;10(1):58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
- van der Veen M. J., Scheven B. A., van Roy J. L., Damen C. A., Lafeber F. P., Bijlsma J. W. In vitro effects of methotrexate on human articular cartilage and bone-derived osteoblasts. Br J Rheumatol. 1996 Apr;35(4):342–349. doi: 10.1093/rheumatology/35.4.342. [DOI] [PubMed] [Google Scholar]