Abstract
OBJECTIVES—The aim of the study was to determine the prevalence and clinical significance of antibodies to individual histone components in systemic sclerosis (SSc). METHODS—Serum samples from patients with limited cutaneous SSc (lSSc; n=42) and diffuse cutaneous SSc (dSSc; n=28) were examined for IgG and/or IgM antibodies to individual histone components and complexes by enzyme linked immunosorbent assay (ELISA). RESULTS—The level of IgG antibody to total histones was significantly higher in lSSc and dSSc than in normal controls. The level of IgM antibody to total histones was significantly higher in lSSc, but not in dSSc, than in normal controls. IgG antibody to total histones tended to be increased in dSSc when compared with that in lSSc. On the other hand, IgM antibody to total histones tended to be increased in lSSc when compared with that in dSSc. Although SSc showed various antihistone specificities, H2B, H2A-H2B, (H2A-H2B)-dsDNA were main antigens recognised by IgG antibodies in both lSSc and dSSc. Although IgM antibodies to H2B and H2A-H2B were also detected in both lSSc and dSSc, serum samples from lSSc patients exhibited highest IgM reactivity with H1. CONCLUSION—SSc may be included among conditions in which heterogeneous antihistone antibodies are produced. IgM antibodies to the most accessible histone H1 may be related to mild clinical features (lSSc) and IgG antibodies to the inner core molecules of native histone such as H2B or complexes including H2B may be associated with severe clinical features (dSSc) in Ssc. Keywords: histone; antibody; systemic sclerosis; enzyme linked immunosorbent assay
Full Text
The Full Text of this article is available as a PDF (124.8 KB).
Figure 1 .
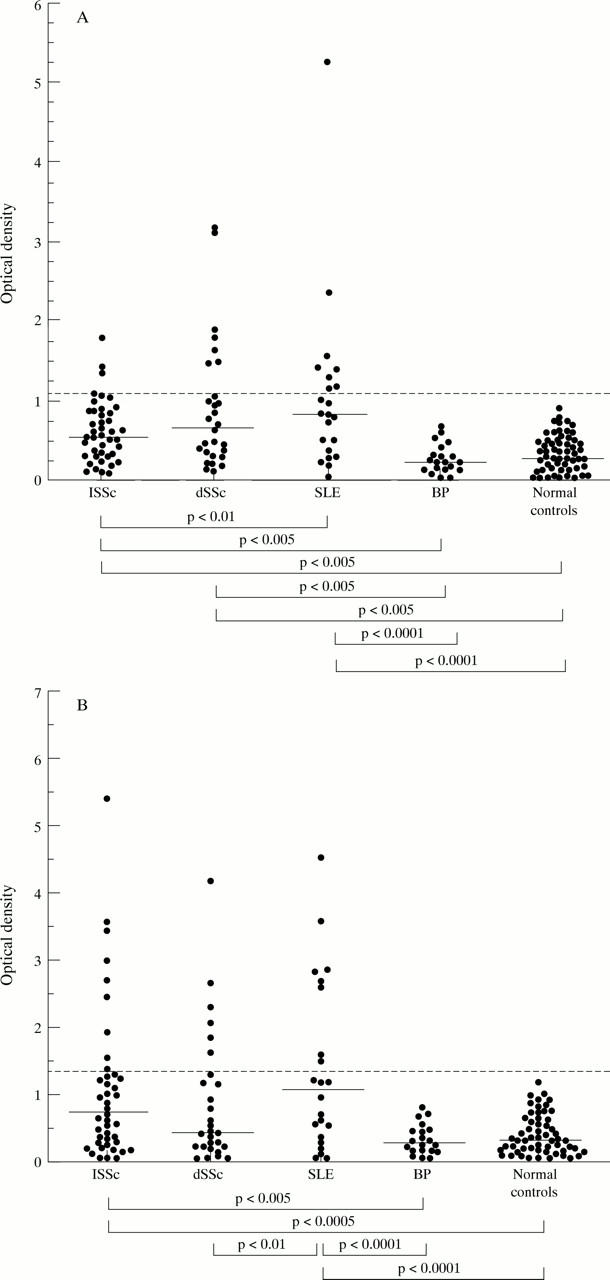
ELISA for antibody to total histones in serum samples from patients with limited cutaneous systemic sclerosis (lSSc) (n=42), diffuse cutaneous systemic sclerosis (dSSc) (n=28), systemic lupus erythematosus (SLE) (n=22), bullous pemphigoid (BP) (n=20), and normal controls (n=57). Horizontal broken line represents the median value +3 SD of antihistone activity in normal control group. Horizontal short bars represent the median values in each group. (A) Serum IgG reactivity to total histones in lSSc, dSSc, SLE, BP, and normal control. (B) Serum IgM reactivity to total histones in lSSc, dSSc, SLE, BP, and normal control.
Figure 2 .
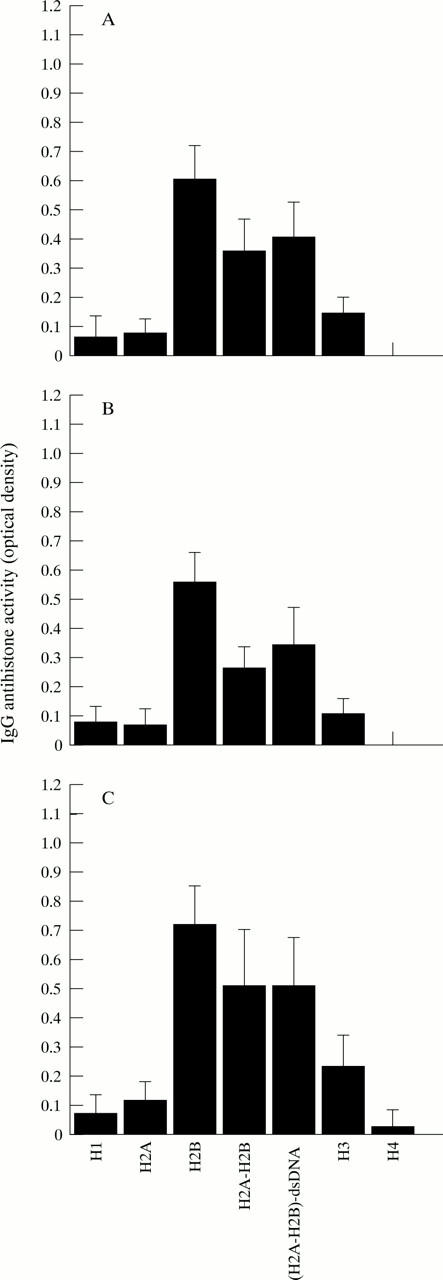
Patterns of IgG reactivity of antihistone antibodies with individual histone components and complexes in patients with systemic sclerosis (SSc). Average OD of IgG reactivity to each histone in normal controls was substracted from the OD (mean (SEM)) of IgG reactivity to each histone in patients. (A) IgG reactivity to histones in patients with SSc (n=70), (B) IgG reactivity to histones in patients with limited cutaneous SSc (lSSc) (n=42), (C) IgG reactivity to histones in patients with diffuse cutaneous SSc (dSSc) (n=28).
Figure 3 .
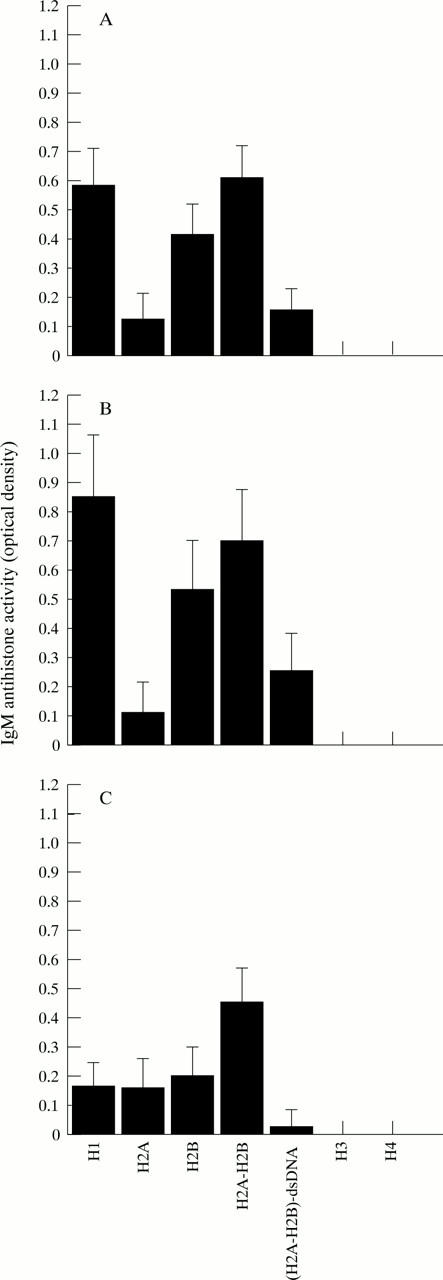
Patterns of IgM reactivity of antihistone antibodies with individual histone components and complexes in patients with systemic sclerosis (SSc). Average OD of IgM reactivity to each histone in normal controls was substracted from the OD (mean (SEM)) of IgM reactivity to each histone in patients. (A) IgM reactivity to histones in patients with SSc (n=70), (B) IgM reactivity to histones in patients with limited cutaneous SSc (lSSc) (n=42), (C) IgM reactivity to histones in patients with diffuse cutaneous SSc (dSSc) (n=28).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D., Van Regenmortel M. H. Nucleosome structure studied with purified antibodies to histones H2B, H3 and H4. FEBS Lett. 1977 Dec 20;85(1):61–64. doi: 10.1016/0014-5793(78)81248-4. [DOI] [PubMed] [Google Scholar]
- Aitcheson C. T., Peebles C., Joslin F., Tan E. M. Characteristics of antinuclear antibodies in rheumatoid arthritis. Reactivity of rheumatoid factor with a histone-dependent nuclear antigen. Arthritis Rheum. 1980 May;23(5):528–538. doi: 10.1002/art.1780230503. [DOI] [PubMed] [Google Scholar]
- Burlingame R. W., Rubin R. L. Drug-induced anti-histone autoantibodies display two patterns of reactivity with substructures of chromatin. J Clin Invest. 1991 Aug;88(2):680–690. doi: 10.1172/JCI115353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzler M. J., Tan E. M. Antibodies to histones in drug-induced and idiopathic lupus erythematosus. J Clin Invest. 1978 Sep;62(3):560–567. doi: 10.1172/JCI109161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblatt D., Bustin M., Sperling R. Heterogeneity in the interaction of chromatin subunits with anti-histone sera visulatized by immuno-electron microscopy. Exp Cell Res. 1978 Mar 1;112(1):1–14. doi: 10.1016/0014-4827(78)90519-0. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C., Black C., Fleischmajer R., Jablonska S., Krieg T., Medsger T. A., Jr, Rowell N., Wollheim F. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988 Feb;15(2):202–205. [PubMed] [Google Scholar]
- Muller S., Soussanieh A., Bouley J. P., Reinbolt J., Van Regenmortel M. H. Localization of two antigenic determinants in histone H4. Biochim Biophys Acta. 1983 Sep 14;747(1-2):100–106. doi: 10.1016/0167-4838(83)90127-9. [DOI] [PubMed] [Google Scholar]
- Parodi A., Drosera M., Barbieri L., Rebora A. Antihistone antibodies in scleroderma. Dermatology. 1995;191(1):16–18. doi: 10.1159/000246474. [DOI] [PubMed] [Google Scholar]
- Portanova J. P., Arndt R. E., Tan E. M., Kotzin B. L. Anti-histone antibodies in idiopathic and drug-induced lupus recognize distinct intrahistone regions. J Immunol. 1987 Jan 15;138(2):446–451. [PubMed] [Google Scholar]
- Rubin R. L., Bell S. A., Burlingame R. W. Autoantibodies associated with lupus induced by diverse drugs target a similar epitope in the (H2A-H2B)-DNA complex. J Clin Invest. 1992 Jul;90(1):165–173. doi: 10.1172/JCI115832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. L., Waga S. Antihistone antibodies in systemic lupus erythematosus. J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):118–126. [PubMed] [Google Scholar]
- Sato S., Fujimoto M., Ihn H., Kikuchi K., Takehara K. Antigen specificity of antihistone antibodies in localized scleroderma. Arch Dermatol. 1994 Oct;130(10):1273–1277. [PubMed] [Google Scholar]
- Sato S., Ihn H., Kikuchi K., Takehara K. Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis. Arthritis Rheum. 1994 Mar;37(3):391–394. doi: 10.1002/art.1780370313. [DOI] [PubMed] [Google Scholar]
- Sato S., Ihn H., Soma Y., Igarashi A., Tamaki T., Kikuchi K., Ishibashi Y., Takehara K. Antihistone antibodies in patients with localized scleroderma. Arthritis Rheum. 1993 Aug;36(8):1137–1141. doi: 10.1002/art.1780360815. [DOI] [PubMed] [Google Scholar]
- Steen V. D., Powell D. L., Medsger T. A., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988 Feb;31(2):196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Wallace D. J., Lin H. C., Shen G. Q., Peter J. B. Antibodies to histone (H2A-H2B)-DNA complexes in the absence of antibodies to double-stranded DNA or to (H2A-H2B) complexes are more sensitive and specific for scleroderma-related disorders than for lupus. Arthritis Rheum. 1994 Dec;37(12):1795–1797. doi: 10.1002/art.1780371213. [DOI] [PubMed] [Google Scholar]


