Abstract
OBJECTIVES—(1)To analyse the in situ expression of adhesion molecules in rheumatoid nodules. (2) To compare the endothelial expression of adhesion molecules in synovial tissue and subcutaneous nodules obtained from the same patients. (3) To compare the expression of adhesion molecules and activation markers on T cell lines from nodules and synovium. METHODS—(1) Immunohistochemical analysis by APAAP technique of E selectin, CD44, ICAM-1, PECAM-1, and VCAM-1 was performed on 10 rheumatoid nodules from seven patients with rheumatoid arthritis (RA); nodules and synovium were simultaneously analysed from three patients. (2) T cell lines were generated from RA nodules (n=7) and synovium (n=7) by interleukin 2 expansion, and subsequently characterised by flow cytometry for surface expression of αEβ7, α4β7, CD44, L selectin, LFA-1a, PECAM-1, and CD30. RESULTS—(1) In rheumatoid nodules, the palisading layer strongly stains for ICAM-1 and PECAM-1, but less pronounced for CD44. VCAM-1 staining was usually negative. ICAM-1 is upregulated in the vessels surrounding the central zone of fibrinoid necrosis. The immunohistological picture in different nodules derived from the same patient was similar. (2) The endothelial expression of adhesion molecules is comparable in RA nodules and synovium on an individual level, except for E selectin, which is overexpressed in nodule endothelium. (3) T cell lines from nodules and synovium display similar adhesion molecule profiles. However, the expression of CD30, a T cell activation marker linked with Th2 subsets, is higher in nodules compared with synovium. CONCLUSION—These data support a recirculation hypothesis of T cells between articular and extra-articular manifestations in RA, although the activation state of the T cells in each of these localisations may differ. Keywords: T cells; adhesion molecules; rheumatoid nodules; rheumatoid synovium
Full Text
The Full Text of this article is available as a PDF (238.8 KB).
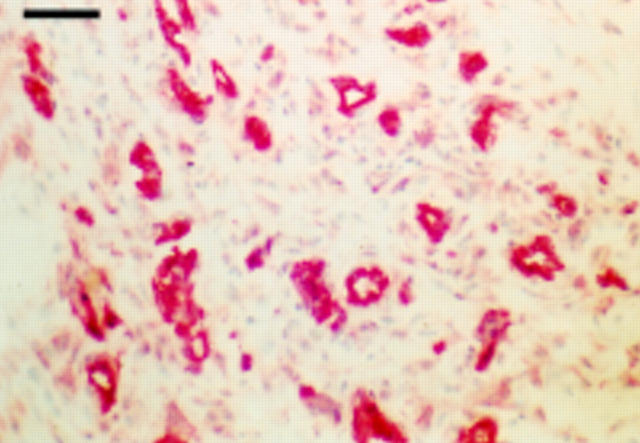
Figure 1 .
Immunostaining of an early onset rheumatoid nodule (removal two weeks after appearance) for PECAM-1. Note impressive vascularisation in the absence of fibrinoid necrosis. Bar=100 µm.
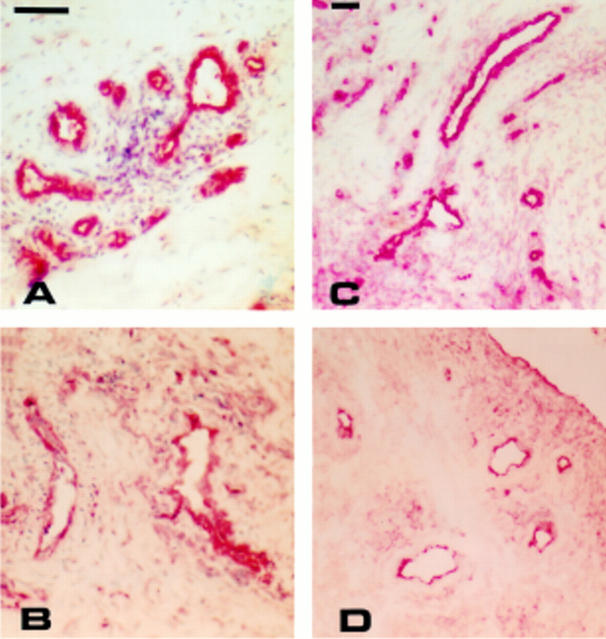
Figure 2 .
Rheumatoid nodules stained for PECAM-1 (A), ICAM-1 (B), E-selectin (C), VCAM-1 (D,E), and CD44 (F). Single staining using APAAP technique counterstained with haematoxylin. A-D: bar=200 µm; E-F: bar=100 µm.
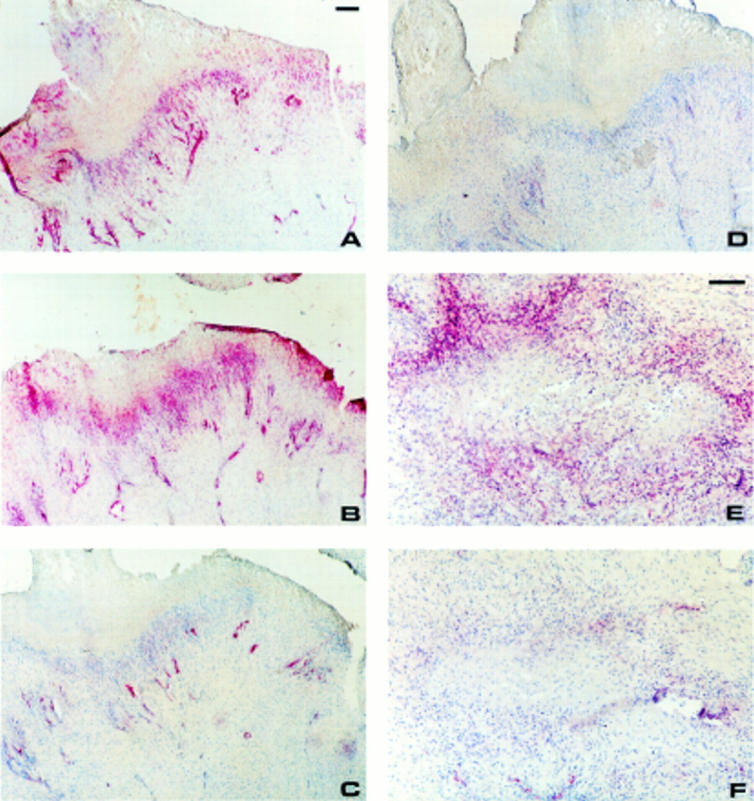
Figure 3 .
Endothelial expression of PECAM-1 (A, C) and ICAM-1 (B, D) in a rheumatoid nodule (A, B) and rheumatoid synovium (C, D). Single staining using APAAP technique counterstained with haematoxylin. Bar=100 µm.
Figure 4 .
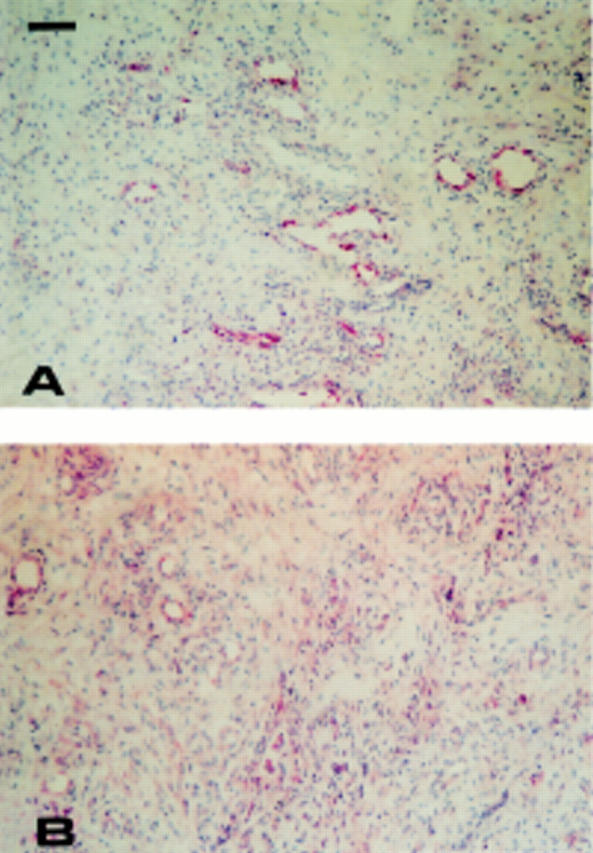
Endothelial expression of E-selectin (A) and VCAM-1 (B) in a rheumatoid nodule. Single staining using APAAP technique counterstained with haematoxylin. Bar=100 µm.
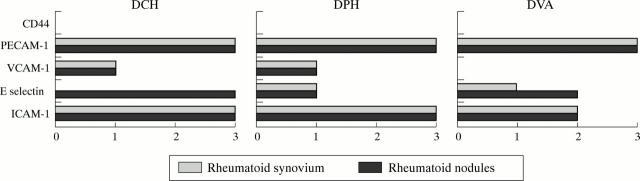
Figure 5 .
Endothelial expression of adhesion molecules in simultaneously obtained rheumatoid nodules and rheumatoid synovium of three patients with rheumatoid arthritis. Data represent semiquantitative scores on endothelium.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Athanasou N. A., Quinn J., Woods C. G., Mcgee J. O. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988 May;47(5):398–403. doi: 10.1136/ard.47.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P. Endothelial-leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keyser F., Elewaut D., Overmeer-Graus J. P., Van den Broek P., Rijnders A. W., Veys E. M. Dominant T cell receptor rearrangements in interleukin 2 expanded lymphocytes from rheumatoid nodules suggest antigen driven T cell activation in situ. J Rheumatol. 1997 Sep;24(9):1685–1689. [PubMed] [Google Scholar]
- De Keyser F., Verbruggen G., Veys E. M., Cuvelier C., Malfait A. M., Benoit D., Elewaut D., Vermeersch J., Heirwegh A. T cell receptor V beta usage in rheumatoid nodules: marked oligoclonality among IL-2 expanded lymphocytes. Clin Immunol Immunopathol. 1993 Jul;68(1):29–34. doi: 10.1006/clin.1993.1090. [DOI] [PubMed] [Google Scholar]
- Del Prete G., De Carli M., Almerigogna F., Daniel C. K., D'Elios M. M., Zancuoghi G., Vinante F., Pizzolo G., Romagnani S. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. FASEB J. 1995 Jan;9(1):81–86. [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Edwards J. C., Wilkinson L. S., Pitsillides A. A. Palisading cells of rheumatoid nodules: comparison with synovial intimal cells. Ann Rheum Dis. 1993 Nov;52(11):801–805. doi: 10.1136/ard.52.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T. M., Simms P. E., Slivnick D. J., Jäck H. M., Fisher R. I. CD30 is a signal-transducing molecule that defines a subset of human activated CD45RO+ T cells. J Immunol. 1993 Sep 1;151(5):2380–2389. [PubMed] [Google Scholar]
- Firestein G. S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996 Nov;39(11):1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Kaye B. R., Kaye R. L., Bobrove A. Rheumatoid nodules. Review of the spectrum of associated conditions and proposal of a new classification, with a report of four seronegative cases. Am J Med. 1984 Feb;76(2):279–292. doi: 10.1016/0002-9343(84)90787-3. [DOI] [PubMed] [Google Scholar]
- Kerstens P. J., Boerbooms A. M., Jeurissen M. E., Fast J. H., Assmann K. J., van de Putte L. B. Accelerated nodulosis during low dose methotrexate therapy for rheumatoid arthritis. An analysis of ten cases. J Rheumatol. 1992 Jun;19(6):867–871. [PubMed] [Google Scholar]
- Lazarovits A. I., Karsh J. Differential expression in rheumatoid synovium and synovial fluid of alpha 4 beta 7 integrin. A novel receptor for fibronectin and vascular cell adhesion molecule-1. J Immunol. 1993 Dec 1;151(11):6482–6489. [PubMed] [Google Scholar]
- Lazarovits A. I., Moscicki R. A., Kurnick J. T., Camerini D., Bhan A. K., Baird L. G., Erikson M., Colvin R. B. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984 Oct;133(4):1857–1862. [PubMed] [Google Scholar]
- Miyasaka N., Sato K., Yamamoto K., Goto M., Nishioka K. Immunological and immunohistochemical analysis of rheumatoid nodules. Ann Rheum Dis. 1989 Mar;48(3):220–226. doi: 10.1136/ard.48.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojcik C. F., Shevach E. M. Adhesion molecules: a rheumatologic perspective. Arthritis Rheum. 1997 Jun;40(6):991–1004. doi: 10.1002/art.1780400602. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Palmer D. G., Hogg N., Highton J., Hessian P. A., Denholm I. Macrophage migration and maturation within rheumatoid nodules. Arthritis Rheum. 1987 Jul;30(7):728–736. doi: 10.1002/art.1780300702. [DOI] [PubMed] [Google Scholar]
- Panayi G. S. The immunopathogenesis of rheumatoid arthritis. Br J Rheumatol. 1993 Mar;32 (Suppl 1):4–14. [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Thomas R., Lipsky P. E. Presentation of self peptides by dendritic cells: possible implications for the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 1996 Feb;39(2):183–190. doi: 10.1002/art.1780390202. [DOI] [PubMed] [Google Scholar]
- Van Boxel J. A., Paget S. A. Predominantly T-cell infiltrate in rheumatoid synovial membranes. N Engl J Med. 1975 Sep 11;293(11):517–520. doi: 10.1056/NEJM197509112931101. [DOI] [PubMed] [Google Scholar]
- Veys E. M., De Keyser F. Rheumatoid nodules: differential diagnosis and immunohistological findings. Ann Rheum Dis. 1993 Sep;52(9):625–626. doi: 10.1136/ard.52.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff M. The rheumatoid nodule. Arthritis Rheum. 1990 Jun;33(6):761–767. doi: 10.1002/art.1780330601. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]