Abstract
OBJECTIVES—The influence of dexamethasone on interleukin 10 (IL10) production and the type 1 (T1)/type 2 (T2) T cell balance found in rheumatoid arthritis (RA) was studied. METHODS—Peripheral blood mononuclear cells (PB MNC) were isolated from 14 RA patients both before and 7 and 42 days after high dose dexamethasone pulse therapy. The ex vivo production of IL10, interferon γ (IFNγ) (T1 cell), and IL4 (T2 cell) by PB MNCs was assessed, along with parameters of disease activity (erythrocyte sedimentation rate, C reactive protein, Visual Analogue Scale, Thompson joint score). In addition, the in vitro effect of dexamethasone (0.02, 0.2, and 2 µM) on PB MNC IL10, IFNγ, and IL4 production was studied. RESULTS—Dexamethasone pulse therapy resulted in a rapid and sustained decrease in RA disease activity. IL10 production increased after dexamethasone treatment and this was sustained for at least six weeks. A transient strong decrease in IFNγ was seen shortly after corticosteroid treatment, while IL4 only decreased slightly. This led to an increased IL-4/IFNγ ratio. In vitro, IL10 production was not detectable, IFNγ and IL4 decreased, but the effect was more pronounced for IFNγ than for IL4, which again resulted in an increased IL4/IFNγ ratio. CONCLUSION—Dexamethasone therapy in RA patients leads to a rapid, clinically beneficial effect. The upregulation of IL10 production may be involved in the prolonged clinical benefit. The strong immunosuppressive effect is most evident in the decrease in IFNγ, and is therefore accompanied by a relative shift towards T2 cell activity. In vitro evaluation showed that this shift in T cell balance was a direct effect of dexamethasone and thus independent of the hypothalamic-pituitary-adrenal axis. Keywords: rheumatoid arthritis; dexamethasone; corticosteroids; T1 T cell; T2 T cell; interferon γ; interleukin 4; interleukin 10
Full Text
The Full Text of this article is available as a PDF (152.7 KB).
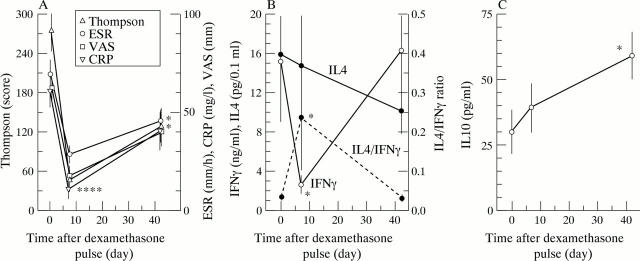
Figure 1 .
(A) RA disease activity; mean (SEM) values of Thompson joint score, erythrocyte sedimentation rate, C reactive protein, and Visual Analogue Scale of 14 RA patients with active disease are given. Patients received 200 mg dexamethasone iv at day 1, 3, and 5. Asterisks indicate a statistical significant difference on day 7 and day 42. (B) Ex vivo IFNγ and IL4 production, as measures for T1 and T2 T cell activity respectively, of PB MNC of 14 RA patients who received 200 mg dexamethasone iv at day 1, 3, and 5. Mean (SEM) values are given. IFNγ and IL4 are shown on the left axis and the average of the individual IL4/IFNγ ratios on the right axis. Asterisks indicate a statistical significant difference on day 7. (C) Ex vivo measured IL10 production of PB MNC of 10 RA patients who received 200 mg dexamethasone iv at day 1, 3, and 5. Mean (SEM) values are given. Asterisk indicates a statistical significant difference on day 42.
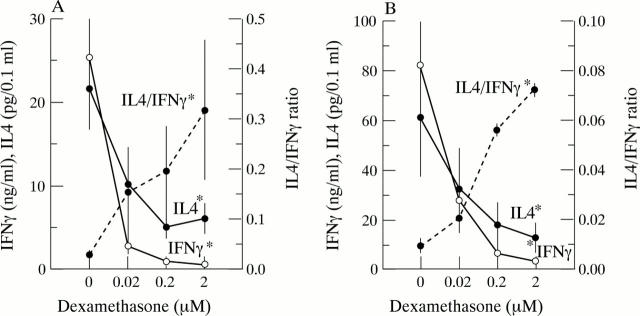
Figure 2 .
(A) In vitro IFNγ and IL4 production, as measures for T1 and T2 T cell activity respectively, of PB MNC obtained from 14 RA patients before dexamethasone pulse therapy, which in vitro have been exposed to dexamethasone. Mean (SEM) values are given. IFNγ and IL4 are shown on the left axis and the average of the individual IL4/IFNγ ratios on the right axis. Asterisks indicate a statistical significant difference at all different dexamethasone concentrations. (B) In vitro IFNγ and IL4 production, as measure for T1 and T2 T cell activity respectively, of PB MNC of seven randomly selected RA patients, in vitro exposed to dexamethasone. Mean (SEM) values are given. IFNγ and IL4 are shown on the left axis and the average of the individual IL4/IFNγ ratios on the right axis. Asterisks indicate a statistical significant difference at all dexamethasone concentrations.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Wong-Staal F., Gallo R. C. Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol. 1984 Jul;133(1):273–276. [PubMed] [Google Scholar]
- Bertouch J. V., Roberts-Thomson P. J., Smith M. D., Woodruff T. G., Brooks P. M., Bradley J. Methylprednisolone infusion therapy in rheumatoid arthritis patients. The effect on synovial fluid lymphocyte subsets and inflammatory indices. Arthritis Rheum. 1986 Jan;29(1):32–38. doi: 10.1002/art.1780290105. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO- and CD45RO+ subsets. J Immunol. 1995 Oct 1;155(7):3322–3328. [PubMed] [Google Scholar]
- Crabtree G. R., Gillis S., Smith K. A., Munck A. Glucocorticoids and immune responses. Arthritis Rheum. 1979 Nov;22(11):1246–1256. doi: 10.1002/art.1780221112. [DOI] [PubMed] [Google Scholar]
- Cush J. J., Splawski J. B., Thomas R., McFarlin J. E., Schulze-Koops H., Davis L. S., Fujita K., Lipsky P. E. Elevated interleukin-10 levels in patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jan;38(1):96–104. doi: 10.1002/art.1780380115. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Araneo B. A. Contrasting effects of glucocorticoids on the capacity of T cells to produce the growth factors interleukin 2 and interleukin 4. Eur J Immunol. 1989 Dec;19(12):2319–2325. doi: 10.1002/eji.1830191221. [DOI] [PubMed] [Google Scholar]
- Dolhain R. J., van der Heiden A. N., ter Haar N. T., Breedveld F. C., Miltenburg A. M. Shift toward T lymphocytes with a T helper 1 cytokine-secretion profile in the joints of patients with rheumatoid arthritis. Arthritis Rheum. 1996 Dec;39(12):1961–1969. doi: 10.1002/art.1780391204. [DOI] [PubMed] [Google Scholar]
- Feldmann M., Brennan F. M., Maini R. N. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Harbuz M. S., Stephanou A., Sarlis N., Lightman S. L. The effects of recombinant human interleukin (IL)-1 alpha, IL-1 beta or IL-6 on hypothalamo-pituitary-adrenal axis activation. J Endocrinol. 1992 Jun;133(3):349–355. doi: 10.1677/joe.0.1330349. [DOI] [PubMed] [Google Scholar]
- Joosten L. A., Lubberts E., Durez P., Helsen M. M., Jacobs M. J., Goldman M., van den Berg W. B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997 Feb;40(2):249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- June C. H., Bluestone J. A., Nadler L. M., Thompson C. B. The B7 and CD28 receptor families. Immunol Today. 1994 Jul;15(7):321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Katsikis P. D., Chu C. Q., Brennan F. M., Maini R. N., Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994 May 1;179(5):1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyson K., McCann S. M. The effect of interleukin-6 on pituitary hormone release in vivo and in vitro. Neuroendocrinology. 1991 Sep;54(3):262–266. doi: 10.1159/000125884. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996 Mar;17(3):138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Norbiato G., Bevilacqua M., Vago T., Clerici M. Glucocorticoids and Th-1, Th-2 type cytokines in rheumatoid arthritis, osteoarthritis, asthma, atopic dermatitis and AIDS. Clin Exp Rheumatol. 1997 May-Jun;15(3):315–323. [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Ramírez F., Fowell D. J., Puklavec M., Simmonds S., Mason D. Glucocorticoids promote a TH2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996 Apr 1;156(7):2406–2412. [PubMed] [Google Scholar]
- Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol. 1995 May;15(3):121–129. doi: 10.1007/BF01543103. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Hernandez-Pando R., Lightman S. L. Hormones, peripherally activated prohormones and regulation of the Th1/Th2 balance. Immunol Today. 1994 Jul;15(7):301–303. doi: 10.1016/0167-5699(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Koops H., Lipsky P. E., Kavanaugh A. F., Davis L. S. Elevated Th1- or Th0-like cytokine mRNA in peripheral circulation of patients with rheumatoid arthritis. Modulation by treatment with anti-ICAM-1 correlates with clinical benefit. J Immunol. 1995 Nov 15;155(10):5029–5037. [PubMed] [Google Scholar]
- Smith M. D., Ahern M. J., Roberts-Thomson P. J. Pulse methylprednisolone therapy in rheumatoid arthritis: unproved therapy, unjustified therapy, or effective adjunctive treatment? Ann Rheum Dis. 1990 Apr;49(4):265–267. doi: 10.1136/ard.49.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijdewint F. G., Kapsenberg M. L., Wauben-Penris P. J., Bos J. D. Corticosteroids class-dependently inhibit in vitro Th1- and Th2-type cytokine production. Immunopharmacology. 1995 Mar;29(2):93–101. doi: 10.1016/0162-3109(94)00048-k. [DOI] [PubMed] [Google Scholar]
- Tabardel Y., Duchateau J., Schmartz D., Marécaux G., Shahla M., Barvais L., Leclerc J. L., Vincent J. L. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery. 1996 Jan;119(1):76–80. doi: 10.1016/s0039-6060(96)80217-0. [DOI] [PubMed] [Google Scholar]
- Thompson P. W., Silman A. J., Kirwan J. R., Currey H. L. Articular indices of joint inflammation in rheumatoid arthritis. Correlation with the acute-phase response. Arthritis Rheum. 1987 Jun;30(6):618–623. doi: 10.1002/art.1780300603. [DOI] [PubMed] [Google Scholar]
- Vacca A., Felli M. P., Farina A. R., Martinotti S., Maroder M., Screpanti I., Meco D., Petrangeli E., Frati L., Gulino A. Glucocorticoid receptor-mediated suppression of the interleukin 2 gene expression through impairment of the cooperativity between nuclear factor of activated T cells and AP-1 enhancer elements. J Exp Med. 1992 Mar 1;175(3):637–646. doi: 10.1084/jem.175.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Pouw-Kraan T., Van Kooten C., Rensink I., Aarden L. Interleukin (IL)-4 production by human T cells: differential regulation of IL-4 vs. IL-2 production. Eur J Immunol. 1992 May;22(5):1237–1241. doi: 10.1002/eji.1830220519. [DOI] [PubMed] [Google Scholar]
- Verhoef C. M., van Roon J. A., Vianen M. E., Bruijnzeel-Koomen C. A., Lafeber F. P., Bijlsma J. W. Mutual antagonism of rheumatoid arthritis and hay fever; a role for type 1/type 2 T cell balance. Ann Rheum Dis. 1998 May;57(5):275–280. doi: 10.1136/ard.57.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weusten B. L., Jacobs J. W., Bijlsma J. W. Corticosteroid pulse therapy in active rheumatoid arthritis. Semin Arthritis Rheum. 1993 Dec;23(3):183–192. doi: 10.1016/s0049-0172(05)80039-3. [DOI] [PubMed] [Google Scholar]
- Wilbrink B., Holewijn M., Bijlsma J. W., van Roy J. L., den Otter W., van Eden W. Suppression of human cartilage proteoglycan synthesis by rheumatoid synovial fluid mononuclear cells activated with mycobacterial 60-kd heat-shock protein. Arthritis Rheum. 1993 Apr;36(4):514–518. doi: 10.1002/art.1780360411. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Haynes D. R., Triantafillou S., Parker A., Gamble J. R., Roberts-Thomson P. J., Ahern M. J., Smith M. D. Effects of pulse methylprednisolone on inflammatory mediators in peripheral blood, synovial fluid, and synovial membrane in rheumatoid arthritis. Arthritis Rheum. 1997 Aug;40(8):1400–1408. doi: 10.1002/art.1780400807. [DOI] [PubMed] [Google Scholar]
- Youssef P. P., Triantafillou S., Parker A., Coleman M., Roberts-Thomson P. J., Ahern M. J., Smith M. D. Effects of pulse methylprednisolone on cell adhesion molecules in the synovial membrane in rheumatoid arthritis. Reduced E-selectin and intercellular adhesion molecule 1 expression. Arthritis Rheum. 1996 Dec;39(12):1970–1979. doi: 10.1002/art.1780391205. [DOI] [PubMed] [Google Scholar]
- Zieg G., Lack G., Harbeck R. J., Gelfand E. W., Leung D. Y. In vivo effects of glucocorticoids on IgE production. J Allergy Clin Immunol. 1994 Aug;94(2 Pt 1):222–230. doi: 10.1016/0091-6749(94)90044-2. [DOI] [PubMed] [Google Scholar]
- van Roon J. A., Verhoef C. M., van Roy J. L., Gmelig-Meyling F. H., Huber-Bruning O., Lafeber F. P., Bijlsma J. W. Decrease in peripheral type 1 over type 2 T cell cytokine production in patients with rheumatoid arthritis correlates with an increase in severity of disease. Ann Rheum Dis. 1997 Nov;56(11):656–660. doi: 10.1136/ard.56.11.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roon J. A., van Roy J. L., Gmelig-Meyling F. H., Lafeber F. P., Bijlsma J. W. Prevention and reversal of cartilage degradation in rheumatoid arthritis by interleukin-10 and interleukin-4. Arthritis Rheum. 1996 May;39(5):829–835. doi: 10.1002/art.1780390516. [DOI] [PubMed] [Google Scholar]
- van den Brink H. R., van Wijk M. J., Geertzen R. G., Bijlsma J. W. Influence of corticosteroid pulse therapy on the serum levels of soluble interleukin 2 receptor, interleukin 6 and interleukin 8 in patients with rheumatoid arthritis. J Rheumatol. 1994 Mar;21(3):430–434. [PubMed] [Google Scholar]