Abstract
OBJECTIVE—To investigate the occurrence of IgA autoantibodies to Ro 52 kDa, Ro 60 kDa and La antigen in serum of patients with primary Sjögren's syndrome (pSS) and systemic lupus erythematosus (SLE). METHODS—Recombinant Ro 52 kDa, Ro 60 kDa and La antigens were used to analyse autoantibodies in serum from 25 patients with pSS, 30 patients with SLE and 20 controls using a semiquantitative immunoblotting approach. RESULTS—Among the patients with pSS, 21 (84%) had detectable IgA autoantibodies to Ro 52 kDa, 13 (52%) to Ro 60 kDa and 20 (80%) to La antigen. The corresponding results for the patients with SLE were 22 (73%), 14 (47%) and 20 (67%), respectively. No IgA autoantibodies against the three antigens were detected in 20 normal controls. A comparison of several clinical features with the titres of IgA antibodies to Ro 52 kDa, Ro 60 kDa and La, revealed a significant relation between IgA anti-Ro 52 and IgA anti-La to sicca (p< 0.05). Semiquantitative data suggest that IgG is the dominating antibody to the three antigens followed by IgM > IgA in both SLE and pSS patients. Specificity studies of IgA autoantibodies with different subfragments of Ro 52 kDa and Ro 60 kDa antigens showed that IgA antibodies did not differ from IgG and IgM in their recognition pattern. CONCLUSION—These results suggest that besides IgM and IgG, IgA autoantibodies are also detected at high frequency in patients with pSS and SLE. Further studies are necessary to evaluate the contribution of these IgA autoantibodies to inflammation as well as their diagnostic value.
Full Text
The Full Text of this article is available as a PDF (377.3 KB).
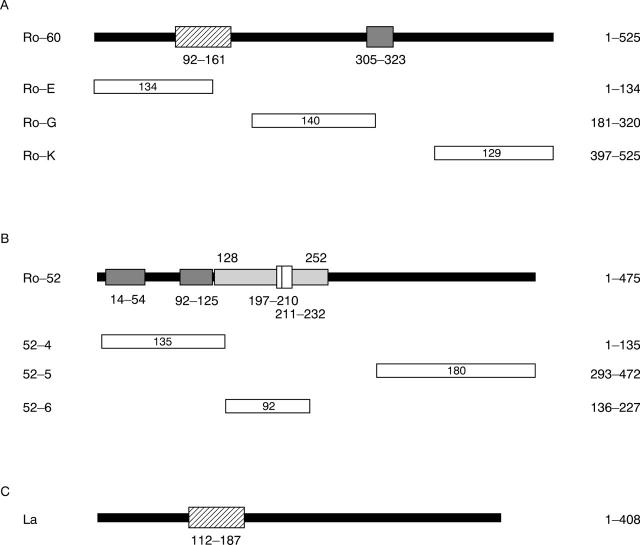
Figure 1 .
Schematic representation of Ro 60 kDa, Ro 52 kDa and La proteins. The hatched boxes indicate the position of RNA binding domains, the dark grey boxes indicate the position of Cys/His clusters and the grey box represents the hydrophilic putative alpha-helical region with an incomplete and a complete leucin zipper indicated by open boxes. Amino acid positions are indicated by numbers. The number of amino acid residues encoded by each clone is indicated inside the corresponding open bar. The position of the clones relative to the deduced Ro 52 kDa, Ro 60 kDa and La amino acid sequence is indicated to the right. (A) Ro 60 kDa protein and three subclones. (B) Ro 52 kDa protein and three subclones. (C) La protein.
Figure 2 .
Illustration of the use of chimeric IgA anti-NIP antibodies to semiquantify immunoblotting data. BSA-NIP conjugate was separated on SDS-PAGE and transferred to a blotting membrane. After incubation with different concentrations of IgA anti-NIP antibodies (0-64 ng/ml) the membrane was incubated with anti-IgA enzyme conjugate and developed with BCIP/NBT for 30 minutes.
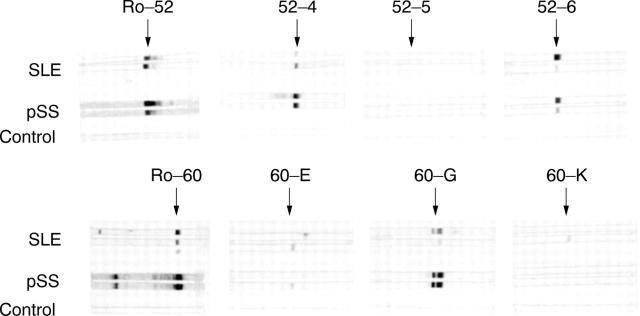
Figure 3 .
Representative recognition pattern of IgA anti-Ro 52 kDa and anti-Ro 60 kDa antibodies to six subfragments in two patients with SLE, two with pSS and one control.
Figure 4 .
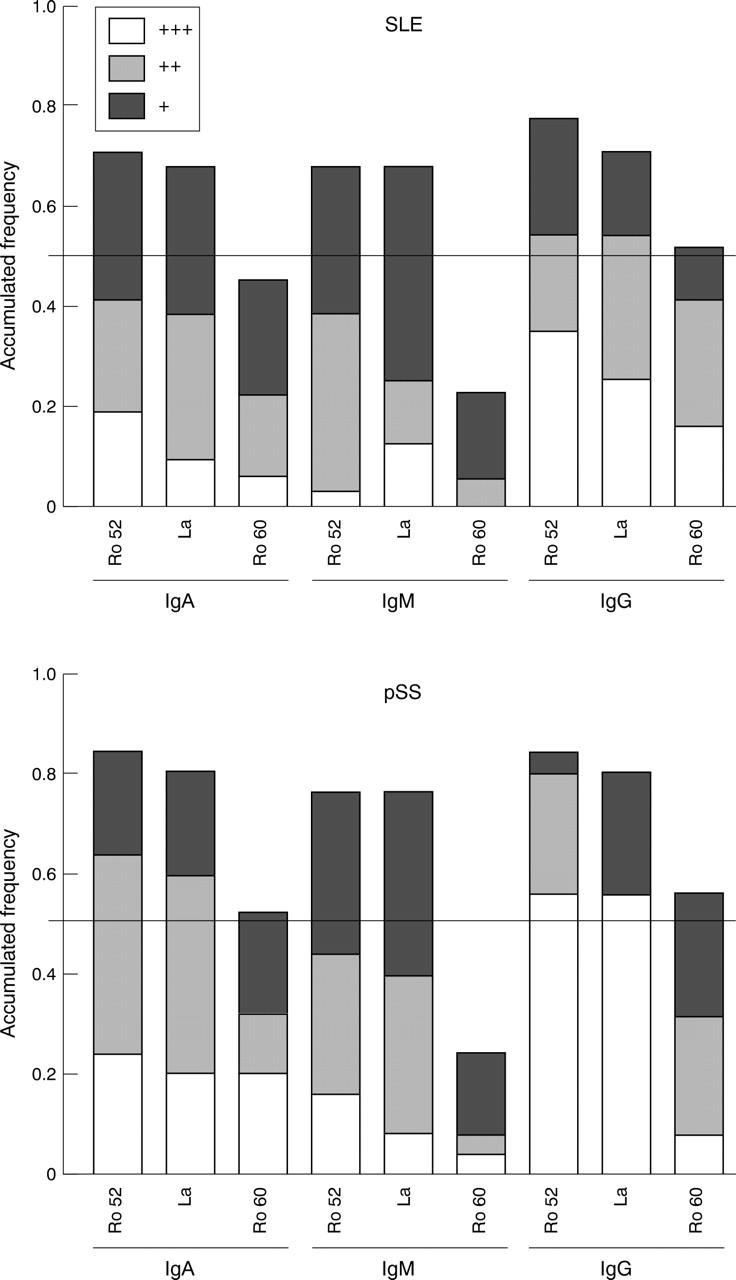
Frequency of serum IgG, IgA and IgM autoantibodies to Ro 60 kDa, Ro 52 kDa and La proteins in pSS and SLE patients by immunoblotting. The intensity of the signal in immunoblotting was semiquantified in relation to serial dilutions of chimeric anti-NIP antibody results. The following groups were established; for IgA 0.25-0.5 µg/ml (+), 0.5-1.0 µg/ml (++) and > 1.0 µg/ml (+++); for IgM 1.50-3.0 µg/ml (+), 3.0-6.0 µg/ml (++) and > 6.0 µg/ml (+++); for IgG 2.0-4.0 µg/ml (+), 4.0-8.0 µg/ml (++) and > 8.0 µg/ml (+++). The horizontal lines indicates median values.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerström B., Björck L. A physicochemical study of protein G, a molecule with unique immunoglobulin G-binding properties. J Biol Chem. 1986 Aug 5;261(22):10240–10247. [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Chetrit E., Gandy B. J., Tan E. M., Sullivan K. F. Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest. 1989 Apr;83(4):1284–1292. doi: 10.1172/JCI114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blange I., Ringertz N. R., Pettersson I. Identification of antigenic regions of the human 52kD Ro/SS-A protein recognized by patient sera. J Autoimmun. 1994 Apr;7(2):263–274. doi: 10.1006/jaut.1994.1020. [DOI] [PubMed] [Google Scholar]
- Bogers W. M., Stad R. K., van Es L. A., Daha M. R. Immunoglobulin A: interaction with complement, phagocytic cells and endothelial cells. Complement Inflamm. 1991;8(5-6):347–358. doi: 10.1159/000463206. [DOI] [PubMed] [Google Scholar]
- Boire G., Gendron M., Monast N., Bastin B., Ménard H. A. Purification of antigenically intact Ro ribonucleoproteins; biochemical and immunological evidence that the 52-kD protein is not a Ro protein. Clin Exp Immunol. 1995 Jun;100(3):489–498. doi: 10.1111/j.1365-2249.1995.tb03728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. Helper T-cell determinants in vaccine design. J Autoimmun. 1989 Jun;2 (Suppl):251–255. doi: 10.1016/0896-8411(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987 Nov 1;166(5):1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. K., Hamel J. C., Buyon J. P., Tan E. M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest. 1991 Jan;87(1):68–76. doi: 10.1172/JCI115003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien P., Soulie E., Johanet C., Abuaf N. Comparisons of double immunodiffusion, ELISA, western blot and CAPE blot for the detection of anti-SSA antibody: study of anti-SSA prevalence in systemic lupus erythematosus. J Autoimmun. 1994 Jun;7(3):379–388. doi: 10.1006/jaut.1994.1027. [DOI] [PubMed] [Google Scholar]
- Conley M. E., Koopman W. J. Serum IgA1 and IgA2 in normal adults and patients with systemic lupus erythematosus and hepatic disease. Clin Immunol Immunopathol. 1983 Mar;26(3):390–397. doi: 10.1016/0090-1229(83)90123-x. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Hughes G. R., Moutsoupoulos H. M. Autoantibodies in the sicca syndrome (primary Sjögren's syndrome). Ann Rheum Dis. 1984 Apr;43(2):243–245. doi: 10.1136/ard.43.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989 Mar;8(3):851–861. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Steitz J. A. The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J. 1989 Mar;8(3):841–850. doi: 10.1002/j.1460-2075.1989.tb03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumpel J. M., Hobbs J. R. Serum immune globulins in Sjögren's syndrome. Ann Rheum Dis. 1970 Nov;29(6):681–683. doi: 10.1136/ard.29.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson I., Rönnelid J., Lundberg I., Jacobson S. H. Occurrence of anti-C1q antibodies in IgA nephropathy. Nephrol Dial Transplant. 1997 Nov;12(11):2263–2268. doi: 10.1093/ndt/12.11.2263. [DOI] [PubMed] [Google Scholar]
- Hall R. P., Stachura I., Cason J., Whiteside T. L., Lawley T. J. IgA-containing circulating immune complexes in patients with igA nephropathy. Am J Med. 1983 Jan;74(1):56–63. doi: 10.1016/0002-9343(83)91118-x. [DOI] [PubMed] [Google Scholar]
- Halse A., Wahren-Herlenius M., Jonsson R. Ro/SS-A- and La/SS-B-reactive B lymphocytes in peripheral blood of patients with Sjögren's syndrome. Clin Exp Immunol. 1999 Jan;115(1):208–213. doi: 10.1046/j.1365-2249.1999.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Itoh Y., Frank M. B. Protein heterogeneity in the human Ro/SSA ribonucleoproteins. The 52- and 60-kD Ro/SSA autoantigens are encoded by separate genes. J Clin Invest. 1991 Jan;87(1):177–186. doi: 10.1172/JCI114968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kelekar A., Saitta M. R., Keene J. D. Molecular composition of Ro small ribonucleoprotein complexes in human cells. Intracellular localization of the 60- and 52-kD proteins. J Clin Invest. 1994 Apr;93(4):1637–1644. doi: 10.1172/JCI117145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauliffe D. P., Lux F. A., Lieu T. S., Sanz I., Hanke J., Newkirk M. M., Siciliano M. J., Sontheimer R. D., Capra J. D. Ro/SS-A and the pathogenic significance of its antibodies. J Autoimmun. 1989 Aug;2(4):375–381. doi: 10.1016/0896-8411(89)90166-2. [DOI] [PubMed] [Google Scholar]
- Meilof J. F., Bantjes I., De Jong J., Van Dam A. P., Smeenk R. J. The detection of anti-Ro/SS-A and anti-La/SS-B antibodies. A comparison of counterimmunoelectrophoresis with immunoblot, ELISA, and RNA-precipitation assays. J Immunol Methods. 1990 Oct 19;133(2):215–226. doi: 10.1016/0022-1759(90)90362-y. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Michaelsen T. E., Aase A., Westby C., Sandlie I. Enhancement of complement activation and cytolysis of human IgG3 by deletion of hinge exons. Scand J Immunol. 1990 Nov;32(5):517–528. doi: 10.1111/j.1365-3083.1990.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Nyman U., Ringertz N. R., Pettersson I. Demonstration of an amino terminal La epitope recognized by human anti-La sera. Immunol Lett. 1989 Jul;22(1):65–71. doi: 10.1016/0165-2478(89)90144-2. [DOI] [PubMed] [Google Scholar]
- O'Brien C. A., Harley J. B. A subset of hY RNAs is associated with erythrocyte Ro ribonucleoproteins. EMBO J. 1990 Nov;9(11):3683–3689. doi: 10.1002/j.1460-2075.1990.tb07580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek R., Pruijn G. J., van Venrooij W. J. Epitope specificity determines the ability of anti-Ro52 autoantibodies to precipitate Ro ribonucleoprotein particles. J Immunol. 1994 Nov 1;153(9):4321–4329. [PubMed] [Google Scholar]
- Peek R., Pruijn G. J., van der Kemp A. J., van Venrooij W. J. Subcellular distribution of Ro ribonucleoprotein complexes and their constituents. J Cell Sci. 1993 Nov;106(Pt 3):929–935. doi: 10.1242/jcs.106.3.929. [DOI] [PubMed] [Google Scholar]
- Pourmand N., Pettersson I. The Zn2+ binding domain of the human Ro 52 kDa protein is a target for conformation-dependent autoantibodies. J Autoimmun. 1998 Feb;11(1):11–17. doi: 10.1006/jaut.1997.0171. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Mansa B. Complement-fixing properties of human IgA antibodies. Alternative pathway complement activation by plastic-bound, but not specific antigen-bound, IgA. Scand J Immunol. 1989 Aug;30(2):175–183. doi: 10.1111/j.1365-3083.1989.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Simons F. H., Pruijn G. J., van Venrooij W. J. Analysis of the intracellular localization and assembly of Ro ribonucleoprotein particles by microinjection into Xenopus laevis oocytes. J Cell Biol. 1994 Jun;125(5):981–988. doi: 10.1083/jcb.125.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobbe R. L., Pruijn G. J., Damen W. G., van der Kemp J. W., van Venrooij W. J. Detection and occurrence of the 60- and 52-kD Ro (SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol. 1991 Oct;86(1):99–105. doi: 10.1111/j.1365-2249.1991.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer R. D. Subacute cutaneous lupus erythematosus: a decade's perspective. Med Clin North Am. 1989 Sep;73(5):1073–1090. doi: 10.1016/s0025-7125(16)30620-4. [DOI] [PubMed] [Google Scholar]
- Stefano J. E. Purified lupus antigen La recognizes an oligouridylate stretch common to the 3' termini of RNA polymerase III transcripts. Cell. 1984 Jan;36(1):145–154. doi: 10.1016/0092-8674(84)90083-7. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Tengnér P., Halse A. K., Haga H. J., Jonsson R., Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody-producing cells in salivary glands from patients with Sjögren's syndrome. Arthritis Rheum. 1998 Dec;41(12):2238–2248. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Wahren M., Ringertz N. R., Pettersson I. IgM and IgG subclass distribution of human anti-Ro/SSA 60 kDa autoantibodies. Scand J Immunol. 1994 Feb;39(2):179–183. doi: 10.1111/j.1365-3083.1994.tb03357.x. [DOI] [PubMed] [Google Scholar]
- Wahren M., Rudén U., Andersson B., Ringertz N. R., Pettersson I. Identification of antigenic regions of the human Ro 60 kDa protein using recombinant antigen and synthetic peptides. J Autoimmun. 1992 Jun;5(3):319–332. doi: 10.1016/0896-8411(92)90146-h. [DOI] [PubMed] [Google Scholar]
- Wehmeyer A., Das P. K., Swaak T., Gebhart W., Kijlstra A. Sjögren syndrome: comparative studies in local ocular and serum immunoglobulin concentrations with special reference to secretory IgA. Int Ophthalmol. 1991 May;15(3):147–151. doi: 10.1007/BF00153916. [DOI] [PubMed] [Google Scholar]
- Whaley K., Webb J., McAvoy B. A., Hughes G. R., Lee P., MacSween R. N., Buchanan W. W. Sjogren's syndrome. 2. Clinical associations and immunological phenomena. Q J Med. 1973 Jul;42(167):513–548. [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli A., Altomonte L., Caricchio R., Galossi A., Mirone L., Scuderi F., Magaro M. Rheumatoid factor in patients with systemic lupus erythematosus. Clin Rheumatol. 1996 May;15(3):312–313. doi: 10.1007/BF02229717. [DOI] [PubMed] [Google Scholar]