Abstract
OBJECTIVE—To define the pattern of mRNA expression of all human matrix metalloproteinases (MMPs) described to date in rheumatoid arthritis (RA) and traumatic synovial membrane, in order to differentiate between a physiological tissue remodelling pattern and that associated with inflammatory tissue destruction. METHODS—Analysis of SwissProt protein and EMBL/GenBank nucleotide sequence banks, protein sequence alignment, reverse transcriptase-polymerase chain reaction and nucleotide sequencing were used. RESULTS—MMP-2 (gelatinase A), MMP-3 (stromelysin-1), MMP-11 (stromelysin-3) and MMP-19 were constitutively expressed. MMP-1 (fibroblast type collagenase), MMP-9 (gelatinase B) and MMP-14 (MT1-MMP) were expressed in all RA, but only in 55-80% of trauma samples. MMP-13 (collagenase-3) and MMP-15 (MT2-MMP) were expressed exclusively in RA (80-90% of the samples). MMP-20 (enamelysin) was absent and MMP-8 (collagenase-2) was rarely found in RA or trauma. All other MMPs (-7, -10, -12, -16, -17) had an intermediate pattern of expression. CONCLUSIONS—Some MMPs without interstitial collagenase activity seem to have a constitutive pattern of expression and probably participate in physiological synovial tissue remodelling. Some MMPs are exclusively associated to RA synovitis, for example, MMP-13, which preferentially degrades type II collagen and aggrecan, and MMP-15, which activates proMMP-2 and proMMP-13 and is involved in tumour necrosis factor α processing. This clear cut rheumatoid/inflammatory MMP profile, more complex than has been previously appreciated, may facilitate inflammatory tissue destruction in RA.
Full Text
The Full Text of this article is available as a PDF (2.6 MB).
Figure 1 .
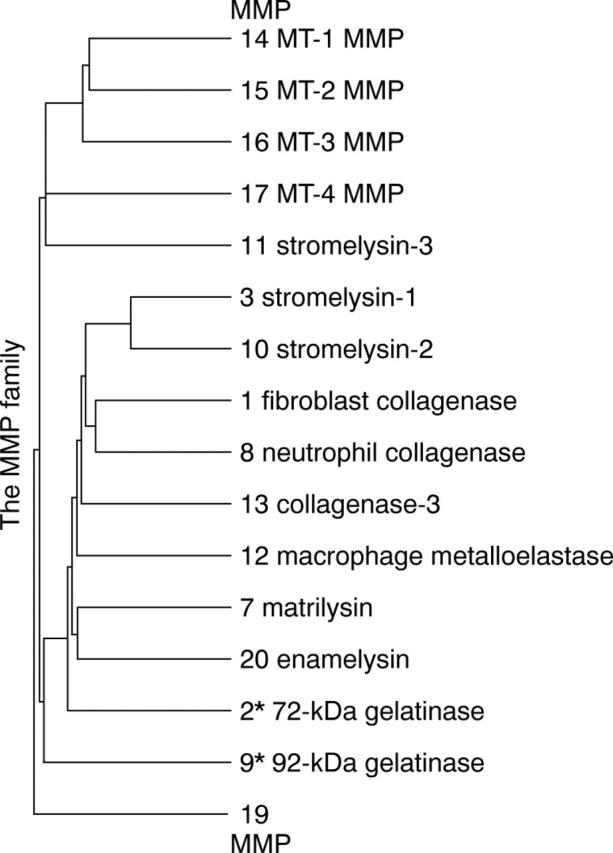
Family tree of the matrix metalloproteinases. Distance along the vertical axis in the family tree is proportional to the difference between sequences. The horizontal distance has no significance.
Figure 2 .
Reverse transcriptase polymerase chain reaction (RT-PCR) control analysis. (A) Ten synovitis tissue samples from RA patients (lanes 1-10). (B) Nine control non-arthritic knee injury samples (lanes 1-9). In both (A) and (B), the upper panel shows mRNA extraction control with β-actin PCR (466 bp) and the lower panel cDNA synthesis control with no RT enzyme (to exclude contamination with genomic DNA), but with regular PCR for β-actin. The expected size of the genomic β-actin would have been 907 bp. Samples are in same order. L indicates 100 bp DNA ladder, starting from 300 bp; N for negative PCR control without sample and C for negative PCR control without sample and primers were performed to exclude contamination of the reagents and found to be negative; P for positive PCR control of β-actin derived from breast cancer cDNA; M for negative cDNA synthesis control without RNA and R for positive cDNA synthesis control with control RNA representing in vitro transcribed RNA from the chloramphenicol acetyltransferase (CAT) gene provided with the kit (523 bp).
Figure 3 .
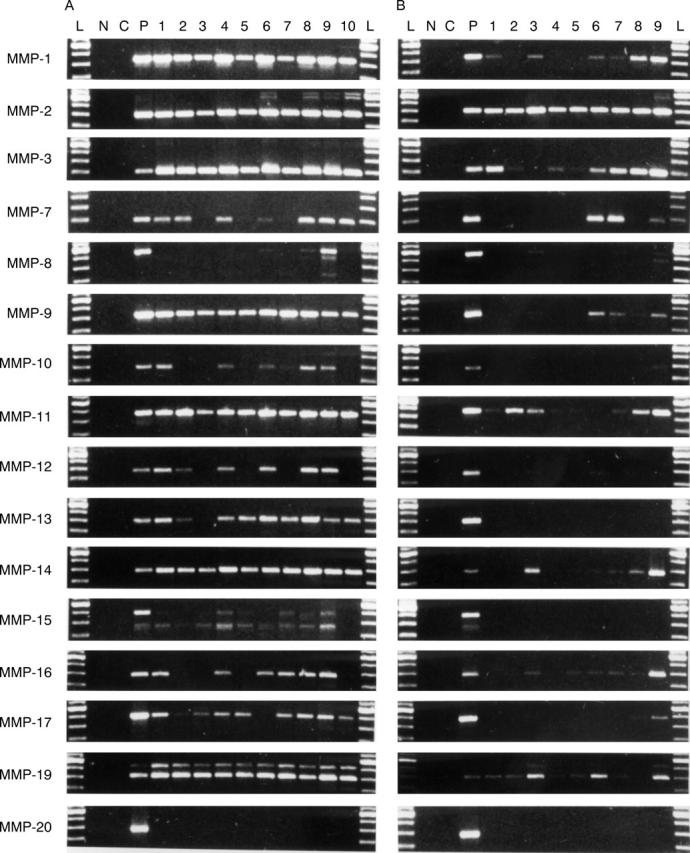
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis of MMP-1 through MMP-20. (A) Ten synovitis tissue samples from RA patients (lanes 1-10). (B) Nine control non-arthritic knee injury samples (lanes 1-9). Samples are in the same order as in figure 2. It should be noted that the weakest bands are not visible in the photographic reproductions. Therefore, all data given in the results section and in table 3 are based on observations made from the original gels and photographs. L indicates 100 bp DNA ladder, starting from 300 bp; N for negative PCR control without sample; C for negative PCR control without sample and primers; and P for positive PCR control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimes R. T., Quigley J. P. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995 Mar 17;270(11):5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in rheumatoid synovium. A transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989 Dec;135(6):1055–1064. [PMC free article] [PubMed] [Google Scholar]
- Chatham W. W., Heck L. W., Blackburn W. D., Jr Lysis of fibrillar collagen by neutrophils in synovial fluid. A role for surface-bound immunoglobulins. Arthritis Rheum. 1990 Sep;33(9):1333–1339. doi: 10.1002/art.1780330905. [DOI] [PubMed] [Google Scholar]
- Cole A. A., Chubinskaya S., Schumacher B., Huch K., Szabo G., Yao J., Mikecz K., Hasty K. A., Kuettner K. E. Chondrocyte matrix metalloproteinase-8. Human articular chondrocytes express neutrophil collagenase. J Biol Chem. 1996 May 3;271(18):11023–11026. doi: 10.1074/jbc.271.18.11023. [DOI] [PubMed] [Google Scholar]
- Cossins J., Dudgeon T. J., Catlin G., Gearing A. J., Clements J. M. Identification of MMP-18, a putative novel human matrix metalloproteinase. Biochem Biophys Res Commun. 1996 Nov 12;228(2):494–498. doi: 10.1006/bbrc.1996.1688. [DOI] [PubMed] [Google Scholar]
- Evanson J. M., Jeffrey J. J., Krane S. M. Human collagenase: identification and characterization of an enzyme from rheumatoid synovium in culture. Science. 1967 Oct 27;158(3800):499–502. doi: 10.1126/science.158.3800.499. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M., Boyle D. L. Mechanisms of methotrexate action in rheumatoid arthritis. Selective decrease in synovial collagenase gene expression. Arthritis Rheum. 1994 Feb;37(2):193–200. doi: 10.1002/art.1780370207. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R., Sorsa T., Konttinen Y. T., Ding Y., Sutinen M., Visser H., van Hinsbergh V. W., Helaakoski T., Kainulainen T., Rönkä H. Matrix metalloproteinase-8 is expressed in rheumatoid synovial fibroblasts and endothelial cells. Regulation by tumor necrosis factor-alpha and doxycycline. J Biol Chem. 1997 Dec 12;272(50):31504–31509. doi: 10.1074/jbc.272.50.31504. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. doi: 10.1093/bioinformatics/5.2.151. [DOI] [PubMed] [Google Scholar]
- Imai K., Ohta S., Matsumoto T., Fujimoto N., Sato H., Seiki M., Okada Y. Expression of membrane-type 1 matrix metalloproteinase and activation of progelatinase A in human osteoarthritic cartilage. Am J Pathol. 1997 Jul;151(1):245–256. [PMC free article] [PubMed] [Google Scholar]
- Imai S., Konttinen Y. T., Jumppanen M., Lindy O., Ceponis A., Kemppinen P., Sorsa T., Santavirta S., Xu J. W., Lopéz-Otín C. High levels of expression of collagenase-3 (MMP-13) in pathological conditions associated with a foreign-body reaction. J Bone Joint Surg Br. 1998 Jul;80(4):701–710. doi: 10.1302/0301-620x.80b4.7952. [DOI] [PubMed] [Google Scholar]
- Kolkenbrock H., Hecker-Kia A., Orgel D., Ulbrich N., Will H. Activation of progelatinase A and progelatinase A/TIMP-2 complex by membrane type 2-matrix metalloproteinase. Biol Chem. 1997 Feb;378(2):71–76. doi: 10.1515/bchm.1997.378.2.71. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Bergroth V., Nordström D., Koota K., Skrifvars B., Hagman G., Friman C., Hämäläinen M., Slätis P. Cellular immunohistopathology of acute, subacute, and chronic synovitis in rheumatoid arthritis. Ann Rheum Dis. 1985 Aug;44(8):549–555. doi: 10.1136/ard.44.8.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen Y. T., Ceponis A., Takagi M., Ainola M., Sorsa T., Sutinen M., Salo T., Ma J., Santavirta S., Seiki M. New collagenolytic enzymes/cascade identified at the pannus-hard tissue junction in rheumatoid arthritis: destruction from above. Matrix Biol. 1998 Dec;17(8-9):585–601. doi: 10.1016/s0945-053x(98)90110-x. [DOI] [PubMed] [Google Scholar]
- Konttinen Y. T., Lindy O., Kemppinen P., Saari H., Suomalainen K., Vauhkonen M., Lindy S., Sorsa T. Collagenase reserves in polymorphonuclear neutrophil leukocytes from synovial fluid and peripheral blood of patients with rheumatoid arthritis. Matrix. 1991 Aug;11(4):296–301. doi: 10.1016/s0934-8832(11)80238-6. [DOI] [PubMed] [Google Scholar]
- Kristo P., Saarelainen R., Fagerström R., Aho S., Korhola M. Protein purification, and cloning and characterization of the cDNA and gene for xylose isomerase of barley. Eur J Biochem. 1996 Apr 1;237(1):240–246. doi: 10.1111/j.1432-1033.1996.0240n.x. [DOI] [PubMed] [Google Scholar]
- Lindy O., Konttinen Y. T., Sorsa T., Ding Y., Santavirta S., Ceponis A., López-Otín C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997 Aug;40(8):1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- Llano E., Pendás A. M., Knäuper V., Sorsa T., Salo T., Salido E., Murphy G., Simmer J. P., Bartlett J. D., López-Otín C. Identification and structural and functional characterization of human enamelysin (MMP-20). Biochemistry. 1997 Dec 9;36(49):15101–15108. doi: 10.1021/bi972120y. [DOI] [PubMed] [Google Scholar]
- Lohi J., Kähäri V. M., Keski-Oja J. Cyclosporin A enhances cytokine and phorbol ester-induced fibroblast collagenase expression. J Invest Dermatol. 1994 Jun;102(6):938–944. doi: 10.1111/1523-1747.ep12384105. [DOI] [PubMed] [Google Scholar]
- Makino Y., Tanaka H., Makino I. Paradoxical derepression of collagenase gene expression by the antirheumatic gold compound aurothiomalate. Mol Pharmacol. 1994 Dec;46(6):1084–1089. [PubMed] [Google Scholar]
- Okada Y., Takeuchi N., Tomita K., Nakanishi I., Nagase H. Immunolocalization of matrix metalloproteinase 3 (stromelysin) in rheumatoid synovioblasts (B cells): correlation with rheumatoid arthritis. Ann Rheum Dis. 1989 Aug;48(8):645–653. doi: 10.1136/ard.48.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendás A. M., Balbín M., Llano E., Jiménez M. G., López-Otín C. Structural analysis and promoter characterization of the human collagenase-3 gene (MMP13). Genomics. 1997 Mar 1;40(2):222–233. doi: 10.1006/geno.1996.4554. [DOI] [PubMed] [Google Scholar]
- Pendás A. M., Knäuper V., Puente X. S., Llano E., Mattei M. G., Apte S., Murphy G., López-Otín C. Identification and characterization of a novel human matrix metalloproteinase with unique structural characteristics, chromosomal location, and tissue distribution. J Biol Chem. 1997 Feb 14;272(7):4281–4286. doi: 10.1074/jbc.272.7.4281. [DOI] [PubMed] [Google Scholar]
- Springman E. B., Angleton E. L., Birkedal-Hansen H., Van Wart H. E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a "cysteine switch" mechanism for activation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):364–368. doi: 10.1073/pnas.87.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolow M. A., Bauzon D. D., Li J., Sedgwick T., Liang V. C., Sang Q. A., Shi Y. B. Identification and characterization of a novel collagenase in Xenopus laevis: possible roles during frog development. Mol Biol Cell. 1996 Oct;7(10):1471–1483. doi: 10.1091/mbc.7.10.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strongin A. Y., Collier I., Bannikov G., Marmer B. L., Grant G. A., Goldberg G. I. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995 Mar 10;270(10):5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- Tardif G., Pelletier J. P., Dupuis M., Hambor J. E., Martel-Pelletier J. Cloning, sequencing and characterization of the 5'-flanking region of the human collagenase-3 gene. Biochem J. 1997 Apr 1;323(Pt 1):13–16. doi: 10.1042/bj3230013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uría J. A., Ståhle-Bäckdahl M., Seiki M., Fueyo A., López-Otín C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997 Nov 1;57(21):4882–4888. [PubMed] [Google Scholar]
- d'Ortho M. P., Will H., Atkinson S., Butler G., Messent A., Gavrilovic J., Smith B., Timpl R., Zardi L., Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997 Dec 15;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]


