Abstract
Transglutaminases (TGases) are defined as enzymes capable of forming isopeptide bonds by transfer of an amine onto glutaminyl residues of a protein. Here we show that the membrane-bound form of the TGase 1 enzyme can also form ester bonds between specific glutaminyl residues of human involucrin and a synthetic analog of epidermal specific ω-hydroxyceramides. The formation of a ≈5-nm-thick lipid envelope on the surface of epidermal keratinocytes is an important component of normal barrier function. The lipid envelope consists of ω-hydroxyceramides covalently linked by ester bonds to cornified envelope proteins, most abundantly to involucrin. We synthesized an analog of natural ω-hydroxyceramides N-[16-(16-hydroxyhexadecyl)oxypalmitoyl]sphingosine (lipid Z). When recombinant human TGase 1 and involucrin were reacted on the surface of synthetic lipid vesicles containing lipid Z, lipid Z was attached to involucrin and formed saponifiable protein–lipid adducts. By mass spectroscopy and sequencing of tryptic lipopeptides, the ester linkage formation used involucrin glutamine residues 107, 118, 122, 133, and 496 by converting the γ-carboxamido groups to lipid esters. Several of these residues have been found previously to be attached to ceramides in vivo. Mass spectrometric analysis after acetonide derivatization also revealed that ester formation involved primarily the ω-hydroxyl group of lipid Z. Our data reveal a dual role for TGase 1 in epidermal barrier formation and provide insights into the pathophysiology of lamellar ichthyosis resulting from defects of TGase 1 enzyme.
Terrestrial vertebrates protect themselves from chemical and physical damage and uncontrolled water loss by maintaining a water-impermeable barrier function of their epidermis. In mammals, this function is essentially accomplished by forming a highly insoluble protein structure on the surface of the corneocytes termed the cornified envelope (CE) and by impeding water diffusion across the stratum corneum by mortaring the corneocytes together by layers of skin-specific lipids (1, 2). These lipids differ in composition from other bilayer-forming lipids found in living cells. Notably, their phospholipid content is lost, and instead they contain increased amounts of free fatty acids, cholesterol and its acyl and sulfate esters, and several classes of ceramides, including epidermal-specific long-chain ω-hydroxy- and ω-hydroxyacylceramides (3). Synthesis of these lipids is initiated in the spinous layer, and they are temporarily stored in lamellar bodies of stratum granulosum, wherein they are arranged as stacks of tetralaminar sheets. Preceding or paralleling the formation of the protein envelope, the contents of the lamellar bodies are extruded into the intercellular space. One component of these lipids is epidermal-specific long-chain ω-hydroxyceramides that become covalently attached onto the outer surface of the CE as a ≈5-nm monomolecular layer. These protein-linked ceramides interdigitate with the intercellular lipid in a comb-like fashion, presumably to stack them into ordered lamellae (4, 5).
All stratified squamous epithelia form a CE protein barrier structure by crosslinking several different proteins into an insoluble macromolecular assembly. Of these proteins, involucrin is a common if not ubiquitous component (6, 7). Several lines of evidence suggest that involucrin is one of the first proteins deposited at or near the membrane surface in the vicinity of desmosomes, to initiate CE formation (7–12). A monomolecular involucrin layer is proposed to function as a scaffold, to which other reinforcing CE proteins such as cystatin α, elafin, small proline-rich proteins, and loricrin are added later by further crosslinking to complete CE assembly (11, 13, 14).
In addition, in the case of the epidermis, it was proposed more than 10 years ago that involucrin may be a protein substrate for the covalent attachment of ceramides by esterification of its glutamate side chains (15, 16). This hypothesis was based on several separate lines of evidence: (i) involucrin is uniquely glutamine/glutamate rich (17); (ii) the ω-hydroxyceramides are ester-linked because they can be removed from corneocytes by saponification with alkali; and (iii) involucrin is ideally located next to the cell membrane, where it could contact ceramides. Indeed, a recent study from this laboratory has afforded robust support for this hypothesis. We isolated from human foreskin epidermis several lipopeptides that contained ceramides ester-linked to glutamine/glutamate residues of identifiable involucrin sequences (18). However, to date there are no data on the biochemical mechanism(s) by which this attachment might occur or which of the ceramide hydroxyl groups engage in ester formation.
Furthermore, involucrin is an excellent substrate for transglutaminases (TGases) both in vitro (19, 20) and in vivo (7). TGases are Ca2+-dependent enzymes, which catalyze the transfer of the γ-carboxy group from protein-bound glutamine to the ɛ-amino group of protein-bound lysine residues or other primary amines. These enzymes are responsible for the crosslinking of CE proteins into a chemically and mechanically resistant protein polymer (21–23). Of the seven known human TGases, four (TGases 1, 2, 3, and X) are expressed in terminally differentiating epithelia such as the epidermis (24, 25). Most TGase 1 activity is bound to plasma membranes, whereas TGases 2 and 3 are cytosolic (6, 8, 21–23). To date, only TGases 1 and 3 have proven roles in CE assembly (21).
Recently, we described an in vitro experimental model system using phosphatidylserine-containing synthetic lipid vesicles (SLV) to explore the role of TGases in the crosslinking of involucrin on or near keratinocyte membranes (28). We found that of these several enzymes, only TGase 1 associates spontaneously with SLV by virtue of its lipid anchors. Interestingly, involucrin also bound to SLV under near-physiological conditions in a dipalmitoyl phosphatidylserine (PS)- and Ca2+-dependent manner and that the plane of the membrane surface sterically directs TGase 1 to use only certain glutamines of involucrin with high specificity. In contrast, in solution assays, TGase 1 and other TGases display little sequence specificity. Here we use this SLV system to bring TGase 1 and involucrin together with an otherwise water-insoluble ω-hydroxyceramide analog, N-[16-(16-hydroxyhexadecyl)oxypalmitoyl]sphingosine (lipid Z). We show that membrane-bound TGase 1, and not other soluble TGases, is capable of chemically attaching lipid Z onto involucrin by esterification.
MATERIALS AND METHODS
Synthesis of Lipid Z.
Lipid Z was synthesized starting from 16-hydroxyhexadecanoic acid, 1,16-hexadecanediol, and d-sphingosine by the method described in the supplementary material on the PNAS web site, together with analytical data of the final product and intermediates (see www.pnas.org).
Production of Recombinant Human TGase 1, TGase 3, and Involucrin Proteins.
These were expressed and purified exactly as described (26). In a separate set of experiments, TGase 1 was freed of its fatty acyl anchors by 1 M NH2OH-HCl (27) to impede its membrane binding and used similarly to the fatty acylated form.
Preparation of SLV.
A mixture of 54 mol % dimyristoylphosphatidylcholine, 15 mol % PS, 30 mol % cholesterol, and 1 mol % lipid Z was made in chloroform/methanol (9:1). This solution was dried down, taken up in aqueous buffer, and dispersed by sonication as before (26). The SLV suspension was equipped with TGase 1 enzyme by incubation at 37°C for 15 min before adding substrates. In some experiments, the deacylated form of TGase 1 or guinea pig liver TGase 2 or dispase-activated recombinant human TGase 3 was added instead. The amount of TGases used was standardized to an activity level of 0.7 pmol per min by using succinylated casein and 14C putrescine as substrates, as described (28).
Reactions of TGases with Involucrin and Lipid Z.
SLV loaded with lipid Z (2 μmol total lipid) were brought to 37°C and 1 mM CaCl2, and 0.6 nmol (40 μg) involucrin was added to make a total volume of 200 μl and incubated for 60 min, if not otherwise stated. The reactions were stopped by the addition of EDTA to 10 mM. For kinetic purposes, some reaction mixtures contained 1 mM putrescine, or alternatively, putrescine to 20 mM was added after running the reaction for 45 min.
Isolation of Lipid-Attached Involucrin Fragments.
After reaction, 0.1 ml 20% SDS in water was added to the samples, and the mixture was vortexed. This mixture was precipitated and washed three times with acetone-triethylamine-acetic acid (90:5:5) to remove the SDS and the unbound lipids. After further washing with acetone, the pellet was dried under vacuum and redissolved in 50 mM Tris⋅HCl (pH 7.5). Resolubilized protein was digested for 16 h with 2 mol % modified trypsin (Boehringer Mannheim). Peptides were resolved on a 250 × 2 mm PrimesphereC4 HPLC column (Phenomenex, Belmont, CA) and separated by using a gradient elution from 75% buffer A (0.1% trifluoroacetic acid in water) to 75% buffer B (50% 2-propanol in acetonitrile) for 40 min. Under these conditions, lipopeptides were retarded but free peptides were eluted with the solvent front (18).
Protein Chemistry Procedures.
Amino acid analysis of hydrolyzed samples (5.7 M HCl at 110°C for 24 h in vacuo) was used to routinely measure amounts of lipopeptides. Aliquots of isolated lipopeptide peaks were attached to a solid support for sequencing (18). Saponification of lipopeptides was done with 1 M KOH in 80% methanol for 2 h at 37°C, after which the solution was acidified and dried, and peptides were recovered by HPLC on a C18 column. The availability of the free hydroxyl groups in positions 1 and 3 on the sphingosine moieties in the lipopeptides was demonstrated by reacting ≈1 nmol of lyophilized lipopeptides in 100 μl in CHCl3/dimethyl acetonide (4:1) in the presence of a trace of camphersulfonic acid for 1 h at 37°C, followed by saponification with 1 M KOH and extraction of the lipids with chloroform/methanol (95:5).
Mass Spectrometry.
Mass analysis was done by using either fast atom bombardment (positive ion mode, magic bullet) or electrospray ionization mass spectrometry for peptides. Spectra were acquired on a JEOL SX102 mass spectrometer fitted with an Analytica electrospray source.
RESULTS
Synthesis of the ω-Hydroxyceramide Analog Lipid Z.
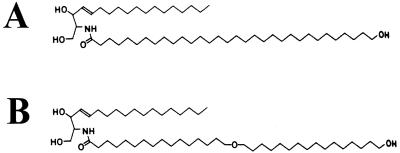
Previously we found that of several types of ceramides present in the skin, only ω-hydroxyceramides having chain lengths of >C28 are ester linked to CE structural proteins (18). Because we have been unable to synthesize natural epidermal ceramides or isolate them in practical amounts, we made an artificial ceramide analog. We synthesized 16-(16-hydroxyhexadecyl)oxyhexandecanoic acid, which is similar in length to natural ceramides containing tetratriacontanoyl (C34) fatty acids and has a terminal (ω) primary alcoholic functional group. This product was linked to a sphingosine base to create the lipid Z ceramide analog (Fig. 1B), which had overall solubility and chromatographic properties similar to pig and human epidermal ceramides (L.N.M. and P.M.S., unpublished work). When present in lipid bilayers, such a long molecule would span a lipid bilayer, allowing the terminal hydroxyl group to be exposed on the side opposite the polar sphingosine moiety.
Figure 1.
Representative structure of a natural ω-hydroxyceramide (A) and the structure of the artificial substrate analog lipid Z (B). The natural and synthetic lipids have similar chain length and differ only by the presence of an (unreactive) ether bond.
Reaction of TGases with Involucrin and Lipid Z on SLV and Recovery of Lipopeptides.
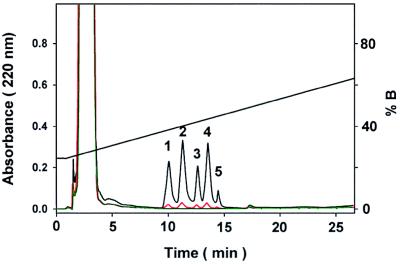
The hydrophobic lipid Z was incorporated into small unilamellar liposome membrane bilayers containing 15% PS, similar to keratinocyte cellular membranes. We showed previously (26) that recombinant human TGase 1, purified from the particulate fraction of insect cells in a baculovirus expression system, reattached spontaneously to SLV by virtue of its fatty acid anchors, which are posttranslationally added in Sf9 insect cells (28). The enzyme was activated by adding Ca2+ to 1 mM. In a previous study, we showed that involucrin attaches spontaneously to SLV under these conditions, and the juxtaposition of TGase 1 and involucrin on the membrane plane of the SLV creates a specific milieu for crosslinking that substantially differs from solution state enzyme-substrate interactions (26). This SLV carrier system was used to react the water-insoluble lipid Z with involucrin by TGase 1. After the reaction and removal of unbound lipids, involucrin was digested with trypsin. Lipopeptide adducts with uniquely hydrophobic properties were selectively isolated on a C4 HPLC column by using highly desorbing conditions and isopropanol gradient elution (18). Under such conditions, free peptides do not bind to the column, and only the lipopeptides are retarded. Eluted lipopeptides were detected by the absorption of peptide bonds at 220 nm. The chemically deacylated form of TGase 1, as well as TGase 2 and TGase 3, neither of which enzymes is attached to SLV membranes (26), did not result in the formation of detectable quantities of ceramide-peptide adducts under identical reaction conditions (data not shown). However, by using membrane-bound TGase 1 enzyme, five peaks were reproducibly separated from the total tryptic digest (Fig. 2). The yields of these peaks directly correlated with TGase 1 amounts in the specific activity range of 0–5 pmol per min. Each peak was subjected to mass analysis, protein sequencing, and quantitation by amino acid analysis after acid hydrolysis.
Figure 2.
Separation by HPLC of lipopeptide adducts from the tryptic digest of involucrin. Involucrin was reacted with SLV containing lipid Z in the presence of TGase 1. Lipid-attached hydrophobic peptides were selectively retained on a C4 column under strongly desorbing solvent conditions (black line). Numbering of peaks refers to the corresponding lipopeptides in Fig. 3 and Tables 1 and 2. In controls for the TGase reaction, no lipopeptides were recovered in the presence of 5 mM EDTA (green line), and lipopeptide amounts were significantly reduced by putrescine (see Fig. 5) or by 20 mM (red line) of the generic TGase inhibitor cystamine.
Mass Spectrometric Analysis of the Recovered Lipopeptides.
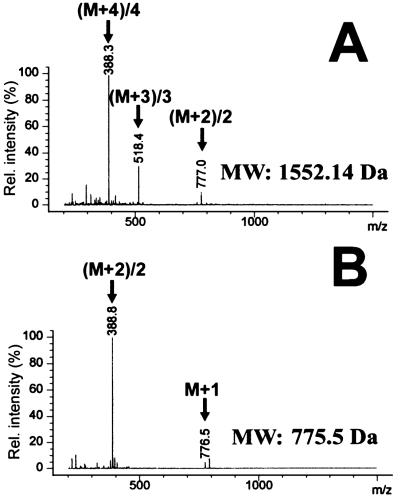
Electrospray ionization mass spectrometry was used to assess the molecular weight of the isolated involucrin lipopeptides before and after alkaline saponification (Fig. 3). All five peaks displayed masses consistent with the presence of only one major detectable mass ingredient. After a 2-h saponification reaction, the masses of each peak decreased by 776.33 to 776.65 atomic mass units (amu), which corresponds to the hydrolysis of a 794.3-Da lipid Z ester (Table 1).
Figure 3.
Analysis of peak 1 of Fig. 2 by electrospray ionization mass spectrometry before (A) and after (B) saponification. Peaks with corresponding masses marked by arrows denote multiple charge states of the peptides used for mass determination. Charge states correspond to m/z = (m + n)/n atomic mass units (n, number of charges). Saponification reduces the molar mass of peptides by 776 amu, indicating the loss by hydrolysis of 1 mol of lipid Z (lipid Z has a mass of 794.3 amu). Deconvoluted masses for all five lipopeptides are listed in Table 1.
Table 1.
Molecular masses of lipopeptide adducts resolved by HPLC (Fig. 2)
| Peak |
Mr
|
ΔMr | |
|---|---|---|---|
| Before and after saponification | |||
| 1 | 1,552.14 | 775.50 | 776.64 |
| 2 | 1,522.12 | 745.47 | 776.65 |
| 3 | 1,833.45 | 1,057.12 | 776.33 |
| 4 | 2,218.86 | 1,442.35 | 776.51 |
| 5 | 2,599.23 | 1,822.88 | 776.35 |
Masses were deconvoluted from electrospray ionization mass spectra before and after saponification.
Specific Glutamine Residues Serve as Substrates for TGase 1-Mediated Esterification in Human Involucrin.
Protein sequencing of each of the tryptic lipopeptide peaks revealed a unique sequence species. However, each sequence differed from the expected native involucrin sequence only on certain Gln residues that were recovered as Glu residues instead (Table 2). Four of the modified Gln residues (Gln107, Gln118, Gln122, Gln133) are located close to each other in the head domain of the protein and are preceded by a Leu residue. In three sequences where there were two consecutive Gln residues, only the second was used, that is GlnGln107,118,133Leu. A fifth Gln residue located in distant sequences toward the end of the central peptide-repeating domain was modified only to a minor extent (Gln496) and was followed by a Val residue. Interestingly, three of these residues (Gln118,122,133) were identified as sites of ceramide attachment in a previous in vivo study (18).
Table 2.
Sequences of lipopeptide adducts resolved by HPLC (Fig. 2)
| Peak | Sequence | Mr | Involucrin sequence |
|---|---|---|---|
| 1 | ELEEEK | 775.8 | Q122 LEEEK |
| 2 | DQELNK | 745.8 | DQQ118LNK |
| 3 | AENPEQELK | 1,057.1 | AENPEQQ107LK |
| 4 | LLDQELDQELVK | 1,442.7 | LLDQQ133LDQELVK |
| 5 | QEAQLELPEQEVGQPK | 1,833.9 | QEAQLELPEQQ496VGQPK |
Boldface letters indicate the modified glutamines participating in the ester formation.
The ω-Hydroxyl Group of Lipid Z Is Preferentially Used in Ester Bond Formation.
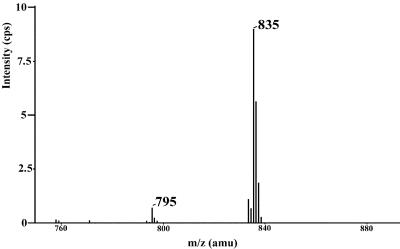
To determine which of the three hydroxyl groups of lipid Z is used by TGase 1 in the esterification reaction, isolated lipopeptides were reacted under acidic conditions with dimethylacetonide (Aldrich), and the modified lipid Z was recovered by subsequent alkaline hydrolysis. By mass spectrometry, the bulk of the lipid was converted to a product of mass of 834 amu, indicating acetonide formation of two closely juxtaposed hydroxyl groups (Fig. 4). Such a derivative could be formed only from lipid Z if the two hydroxyls in positions 1 and 3 on the sphingosine moiety were vacant and not esterified to the peptides. Approximately 90% of the lipid appeared as acetonide derivative by mass spectrometric analysis, whereas the rest either was not converted or was hydrolyzed during processing. Similar conversion yields were obtained by using free lipid Z instead of lipopeptides, showing that complete conversion is not achievable by this method or that some acetonide is hydrolyzed during isolation. As additional controls, when lipid Z was replaced with palmitoylsphingosine or 16-hydroxypalmitoylsphingosine in SLV membranes, no involucrin-adduct formation occurred (data not shown), indicating that the hydroxyl group on the end of an acyl chain that is long enough to span the lipid bilayer membrane is a sine qua non for the esterification reaction.
Figure 4.
Mass spectrometry of lipid Z after acetonide formation of its peptide adducts and subsequent alkaline hydrolysis. Most of the lipid Z (M + H+ = 795 amu) was recovered as its acetonide derivative (M + H+ = 835 amu), indicating that the hydroxyl groups of the sphingosine moiety in lipid Z are not involved in ester bond formation with involucrin.
Kinetics of Lipid Z Esterification of Involucrin by TGase 1.
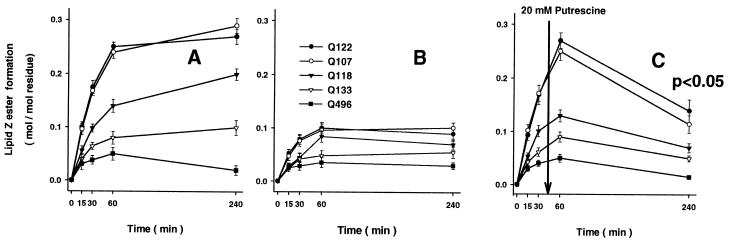
As much as 25 mol % of the lipid Z substrate could be incorporated into SLV without interference with SLV stability or binding of involucrin and TGase 1. The rate of lipid Z incorporation into involucrin remained linear up to this level, thereby indicating high KM (>100 mol %). However, for practical reasons we routinely used SLV formulations with 1 mol % lipid Z, in which the incorporation rate was 2.2 ± 0.3 fmol of lipid Z per s at 0.7 pmol putrescine per min specific TGase 1 activity. Nevertheless, under all experimental conditions tested, we could achieve only partial esterification of the five reactive Gln residues (Fig. 5A). By using amounts of involucrin that were determined not to saturate the binding capacity of the SLV (0.3 nmol protein per μmol lipid; see ref. 26), a maximum of 0.1–0.3 mol of lipid ester formation was possible per mol of Gln residues 107, 118, 122, and 133, and <0.05 mol/mol for Gln 496 in the 4-h reaction. Addition of further TGase 1 enzyme did not increase the extent of esterification. In a series of controls, as expected, lipid Z esterification was completely inhibited in the presence of 5 mM EDTA or 20 mM of the TGase active site inhibitor cystamine (Fig. 2). Addition of up to 2% of the water-soluble primary alcohol 1-hexanol did not significantly (P = 0.05) inhibit formation of lipid Z ester (data not shown). Further, inclusion of 1 mM putrescine as a competitive TGase amine cosubstrate inhibited ester formation of each reactive Gln residue by 2- to 3-fold (Fig. 5B). Likewise, when 20 mM putrescine was added at 45 min during the reaction, there was about a 2-fold and time-dependent reduction in ester yields as compared with 1-h levels (Fig. 5C).). Together, these data establish that the esterification of lipid Z onto involucrin is a membrane-bound TGase 1-dependent reaction: the reaction rate depends directly on the amount of enzyme, and inhibitors of TGase activity reduced or abolished ester bond formation (Fig. 2).
Figure 5.
Inhibitory effect of putrescine on glutaminyl ester formation by TGase 1. Amounts of individual lipopeptides were quantitated by amino acid analysis after isolation by C4 HPLC chromatography. Yields were related to the total of reactive Gln residues. (A) Ester modification of reactive glutamines without inhibitor. (B) Putrescine (1 mM) inhibited the formation of lipid adducts. (C) Putrescine (20 mM) significantly (P < 0.01) reduced the amount of ester linkages when added to the reaction at 45 min, showing the availability of ester linkages for TGase-mediated aminolysis. Values (mean ± SEM) are from five separate experiments.
DISCUSSION
A Dual Role for TGase 1.
TGases are well known to perform a two-step reaction, first activating a protein-bound glutamine residue through a thioester intermediate, followed by the transfer of the glutaminyl moiety to an acceptor amine group, usually the ɛ-NH2 group of a protein-bound lysine residue [thereby forming an Nɛ(γ-glutamyl)lysine isopeptide bond] or a polyamine (21–23). Typically, TGases are responsible for the crosslinking of proteins to form stable insoluble macromolecular assemblies that are used for many purposes in biology. In the absence of an acceptor amine, the acyl transfer may occur onto water, resulting in deamidation of the protein-bound glutamine residue. In addition, early studies have shown, by using model peptide substrates, that TGase 2 may also form an ester linkage by transferring the glutaminyl moiety to a primary alcohol (29, 30), but heretofore the biological occurrence or significance of this reaction has not been demonstrated. A glutamine residue in a protein represents an intrinsically activated higher free-energy derivative of glutamic acid, and the release of ammonia from its γ-carboxamido group provides sufficient free energy to drive the reaction to form isopeptide or ester bonds.
Recently, we showed that ω-hydroxyceramides with long (C28–C36) acyl chains are ester linked to glutamine and glutamate residues of a number of CE structural proteins, most notably involucrin (18). In this way, such ceramides form the lipid-bound envelope and contribute to the barrier function of the epidermis.
In this study, we have demonstrated that the membrane-bound form of the TGase 1 enzyme is in fact capable of forming ester linkages between ceramides and involucrin. Ceramides are insoluble in an aqueous environment but can be dissolved in membranes. We have used our recent system with SLV membranes of PS content similar to that expected for keratinocyte plasma membranes (26). The SLV serve as a vehicle for TGase 1 because the enzyme harbors palmitoyl and myristoyl anchors. Furthermore, in the presence of micromolar concentrations of Ca2+, these SLV bind involucrin. On raising the Ca2+ concentration, the TGase 1 enzyme activates with high specificity Gln107,118,122,133,and496 of involucrin, apparently because of a favorable steric alignment on the SLV surface (26). In this study, we added a synthetic ceramide (lipid Z) to this reaction, which has dimensions and chemistry typical of those of epidermal ω-hydroxyceramides. Synthesis of a ceramide of sufficient length posed a daunting problem, especially because ω-hydroxyl derivatives of fatty acids longer than tetracosanoic (C24) have not been reported in organic chemistry. Thus we made lipid Z with an ether derivative of an ω-hydroxyacyl chain having a net length equivalent approximately to a naturally occurring epidermal C34 ceramide (23). By using the SLV carrier system, the TGase 1 enzyme could ester link this derivative onto involucrin. Notably, other soluble TGases were not capable of catalyzing this attachment reaction onto involucrin.
We note that the amine cosubstrate putrescine reduced the rate of ester formation as expected (Fig. 5B) and also caused detectable decay of ester products with time (Fig. 5C). We attribute this phenomenon to aminolysis of ester bonds by putrescine. This reaction could occur only if the conversion of the thioacyl enzyme intermediate into an ester bond is an effectively reversible process, and the amine can deplete the acyl-enzyme intermediate because of its lower free energy (31). Therefore, ester bonds formed on involucrin are potentially susceptible to aminolysis by lysines on proteins, resulting in the formation of the more stable (lower free energy) “classical” Nɛ(γ-glutamyl)lysine isopeptide crosslink.
Esterification of Specific Glutamine Residues of Involucrin: Comparison with in Vivo Studies.
In this way, five different Gln residues of involucrin were esterified with high specificity and modest efficiency (Fig. 3; Table 1). Four of these are located in the phylogenetically ancient head domain of involucrin, of which Gln107,118,122 have been highly conserved in dog, rat, pig, and human (32). Moreover, three of the residues (Gln118,122,133) tagged in this in vitro study were observed in our previous in vivo study (18). These observations indicate that the head domain of involucrin has been conserved because several of its Gln residues are favorably aligned for ceramide attachment. In this study, we also noted minor reaction of Gln496 located in distant repeating sequences in the evolutionarily more recent part of involucrin. However, the low yield of esterification of Gln496 in vitro contrasts with its near quantitative use for amine attachment (26). Although the stoichiometry of esterification in vitro was low (Fig. 5), we have no direct information on the efficiency with which these residues are labeled in vivo. However, assuming involucrin of dimensions 45 × 1.5 nm (15) forms a monomolecular layer on the cell periphery (14) and based on our earlier estimate of one ceramide moiety per 40 nm2 (18), this means there is about 2 mol of ceramide per mol of involucrin. Thus, for steric reasons each of the several potentially reactive Gln residues is likely to be labeled substoichiometrically in vivo.
Our previous in vivo study also identified other head domain Gln residues of involucrin, which we did not see esterified in this study (18). Further work will be necessary to characterize these residues. It is possible they are labeled by other processed forms of the membrane-anchored TGase 1 enzyme, or perhaps novel TGases. Likewise, further work will be necessary to explore the ceramide esterification of Gln residues of the other cell-peripheral CE proteins, such as envoplakin and periplakin, seen in vivo.
In addition, the in vivo study identified ceramide esterification of several Glu residues. Currently we do not know how these Glu residues may be activated to a high free-energy state to drive ester bond formation.
A Model for CE Assembly.
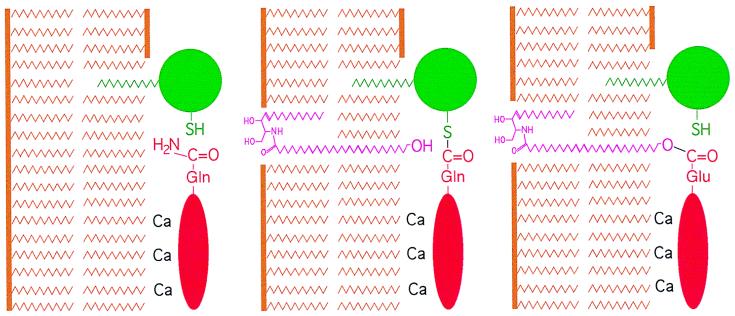
Epidermal-specific ceramides are not only unique but are also intriguing, because their C28–36 acyl chains are minimally or comfortably long enough to span a plasma membrane bilayer, so that the ω-hydroxyl group can be located on the side opposite the sphingosine moiety. Here we found that the alkyl chain of lipid Z, which is longer than the thickness of a lipid bilayer membrane, allowed the specific use of the ω-hydroxyl for esterification to involucrin, whereas an ω-hydroxyceramide with C16 fat chain did not. Moreover, only the ω-hydroxyl group, rather than the sphingosine hydroxyls, were used for crosslinking (Fig. 4.). Our in vitro findings agree with recent data suggesting that in vivo ω-hydroxyglucosyceramides become ester linked through their ω-hydroxyl group before deglycosylation of their sphingosine moieties (33–35). In this way, only the ω-hydroxyl would be accessible for esterification by the TGase 1 enzyme onto adjacent involucrin and the other early CE or membrane constituents such as envoplakin and periplakin (Fig. 6). These events occur during late stages of keratinocyte terminal differentiation and cell death, corresponding to the uppermost granular cells, presumably coincident with the extrusion of lamellar body contents. If so, this may mean that the ceramide lipid envelope is attached before completion of protein envelope assembly involving massive deposition of loricrin and small proline rich proteins.
Figure 6.
Model for the reaction of the ω-hydroxyl group of epidermal ceramides. The TGase 1 enzyme (green) and involucrin (red) are bound to cellular membrane bilayers (brown) by acyl lipid adducts and Ca2+ ions, respectively (Left). The catalytic membranes might be either the plasma membrane or the limiting membranes of lamellar bodies (33). The weak nucleophil ω-hydroxyl group is exposed adjacent to the TGase 1-involucrin acyl-enzyme intermediate (Center), resulting in a high likelihood of nucleophilic attack of the thioester bond (Right), resulting in the release of TGase 1 and formation of an ester linkage.
Conclusions: Implications for Ichthyosis Diseases.
It is to be expected that perturbation of the lipid envelope formation involving ceramide attachment should interfere with complete CE assembly and also with the organization and stability of the intercellular lamellae. The probable negative outcome is an ichthyosiform disorder resulting from the diminished barrier function (36–38). Disorder could occur by inability to correctly synthesize the ceramide lipids as in Refsum’s disease [phytanic acid accumulation caused by phytanoyl-CoA hydroxylase deficiency (36–39)] and Sjögren–Larsson’s syndrome [pathological lipid metabolism caused by fatty aldehyde dehydrogenase deficiency (40)]. Alternatively, disease could occur by the inability to correctly attach the ceramides. Notably, loss of TGase 1 activity causes the often devastating life-threatening disease lamellar ichthyosis (41, 42). Our present observations indicate that an essential role of this enzyme is to attach ceramides. The expected diminution of barrier function is consistent with the clinical and pathological observations of the human disease, as well as of the mouse knockout model (43). Interestingly, pathology of lamellar ichthyosis is largely restricted to the epidermis and its appendages, which form lipid envelopes; internal stratified squamous epithelia that normally do not make lipid envelopes yet express abundant levels of TGase 1 for the formation of their protein envelopes are not notably affected (37, 38, 42, 44). Thus, we might conclude that the severity of the LI phenotype is contributed by the inability of the TGase 1 enzyme to assemble both the lipid envelope by ester bond formation and the protein envelope by crosslinking of CE proteins.
Supplementary Material
Acknowledgments
We are indebted to Dr. Lewis Pannell and Melinda Owen for mass spectrometry and Dr. Vince Pozsgai and Dr. Terrence Burke for methodological support and advice.
ABBREVIATIONS
- amu
atomic mass unit
- CE
cornified envelope
- HPLC
high performance liquid chromatography
- lipid Z
N-[16-(16-hydroxyhexadecyl)oxypalmitoyl]sphingosine
- PS
dipalmitoyl phosphatidylserine
- SLV
synthetic lipid vesicles
- TGase
transglutaminase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Downing D T, Stewart M E, Wertz P W, Strauss J S. In: Dermatology in General Medicine. Fitzpatrick T B, Eisen A Z, Wolff K, Freedberg I M, Austen K F, editors. New York: McGraw–Hill; 1993. pp. 210–221. [Google Scholar]
- 2.Ponec M. In: The Keratinocyte Handbook. Leigh I M, Lane E, Watt F M, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 351–363. [Google Scholar]
- 3.Elias P M, Menon G K. In: Skin Lipids, Advances in Lipid Research. Elias P M, editor. Vol. 24. San Diego: Academic; 1991. pp. 1–26. [DOI] [PubMed] [Google Scholar]
- 4.Wertz P W. Experientia Suppl. 1997;78:227–237. [Google Scholar]
- 5.Wertz P W, Downing D T. In: Physiology, Biochemistry and Molecular Biology of the Skin. Goldsmith L A, editor. Vol. 1. Oxford: Oxford Univ. Press; 1991. pp. 205–236. [Google Scholar]
- 6.Simon M. In: The Keratinocyte Handbook. Leigh I M, Lane E B, Watt F M, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 275–292. [Google Scholar]
- 7.Steinert P M, Marekov L N. J Biol Chem. 1997;272:2021–2030. doi: 10.1074/jbc.272.3.2021. [DOI] [PubMed] [Google Scholar]
- 8.Rice R H, Green H. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 9.Simon M, Green H. Cell. 1984;36:827–834. doi: 10.1016/0092-8674(84)90032-1. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe M B, Murthy S, Eckert R L. J Invest Dermatol. 1993;100:3–9. doi: 10.1111/1523-1747.ep12349857. [DOI] [PubMed] [Google Scholar]
- 11.Steinert P M. Cell Death Different. 1995;2:33–40. [PubMed] [Google Scholar]
- 12.Ishida-Yamamoto A, Kartasova T, Matsuo S, Kuroki T, Iizuka H. J Invest Dermatol. 1997;108:12–16. doi: 10.1111/1523-1747.ep12285611. [DOI] [PubMed] [Google Scholar]
- 13.Yaffe M B, Beegen H, Eckert R L. J Biol Chem. 1992;267:12233–12238. [PubMed] [Google Scholar]
- 14.Jarnik M, Simon M N, Steven A C. J Cell Sci. 1998;111:1051–1060. doi: 10.1242/jcs.111.8.1051. [DOI] [PubMed] [Google Scholar]
- 15.Wertz P W, Madison K C, Downing D T. J Invest Dermatol. 1989;92:109–111. doi: 10.1111/1523-1747.ep13071317. [DOI] [PubMed] [Google Scholar]
- 16.Downing D T. J Lipid Res. 1992;33:301–313. [PubMed] [Google Scholar]
- 17.Eckert R L, Green H. Cell. 1986;46:583–589. doi: 10.1016/0092-8674(86)90884-6. [DOI] [PubMed] [Google Scholar]
- 18.Marekov L N, Steinert P M. J Biol Chem. 1998;273:17763–17770. doi: 10.1074/jbc.273.28.17763. [DOI] [PubMed] [Google Scholar]
- 19.Simon M, Green H. J Biol Chem. 1988;263:18093–18098. [PubMed] [Google Scholar]
- 20.Etoh Y, Simon M, Green H. Biochem Biophys Res Commun. 1986;136:51–56. doi: 10.1016/0006-291x(86)90875-2. [DOI] [PubMed] [Google Scholar]
- 21.Melino, G., Candi, E. & Steinert, P. M. (1999) Methods Enzymol., in press. [DOI] [PubMed]
- 22.Greenberg C S, Birckbichler P J, Rice R H. FASEB J. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 23.Reichert U, Michel S, Schmidt R. In: Molecular Biology of the Skin. The Keratinocyte. Darmon M, Bloomberg M, editors. San Diego: Academic; 1993. pp. 107–150. [Google Scholar]
- 24.Kim H C, Idler W W, Kim I G, Han J H, Chung S I, Steinert P M. J Biol Chem. 1991;266:536–539. [PubMed] [Google Scholar]
- 25.Aeschlimann D, Koeller M K, Allen-Hoffmann B L, Mosher D F. J Biol Chem. 1998;273:3452–3460. doi: 10.1074/jbc.273.6.3452. [DOI] [PubMed] [Google Scholar]
- 26.Nemes Z, Marekov L N, Steinert P M. J Biol Chem. 1999;274:11013–11021. doi: 10.1074/jbc.274.16.11013. [DOI] [PubMed] [Google Scholar]
- 27.Steinert P M, Kim S Y, Chung S I, Marekov L N. J Biol Chem. 1996;271:26242–26250. doi: 10.1074/jbc.271.42.26242. [DOI] [PubMed] [Google Scholar]
- 28.Candi E, Melino G, Lahm A, Ceci R, Rossi A, Kim I G, Ciani B, Steinert P M. J Biol Chem. 1998;273:13693–13702. doi: 10.1074/jbc.273.22.13693. [DOI] [PubMed] [Google Scholar]
- 29.Gross M, Folk J E. J Biol Chem. 1974;249:3021–3025. [PubMed] [Google Scholar]
- 30.Parameswaran K N, Lorand L. Biochemistry. 1981;20:3703–3711. doi: 10.1021/bi00516a006. [DOI] [PubMed] [Google Scholar]
- 31.Parameswaran K N, Cheng X F, Chen E C, Velasco P T, Wilson J H, Lorand L. J Biol Chem. 1997;272:10311–10317. doi: 10.1074/jbc.272.15.10311. [DOI] [PubMed] [Google Scholar]
- 32.Green H, Djian P. Mol Biol Evol. 1992;9:977–1017. doi: 10.1093/oxfordjournals.molbev.a040775. [DOI] [PubMed] [Google Scholar]
- 33.Uchida Y, Sidransky E, Ginns E I, Elias P M, Holleran W M. J Invest Dermatol. 1999;112:543. [Google Scholar]
- 34.Doering T, Holleran W M, Potratz A, Vielhaber G, Elias P M, Suzuki K, Sandhoff K. J Biol Chem. 1999;274:11038–11045. doi: 10.1074/jbc.274.16.11038. [DOI] [PubMed] [Google Scholar]
- 35.Doering T, Proia R L, Sandhoff K. FEBS Lett. 1999;447:167–170. doi: 10.1016/s0014-5793(99)00274-4. [DOI] [PubMed] [Google Scholar]
- 36.Williams M L, Elias P M. Dermatol Clin. 1987;5:155–178. [PubMed] [Google Scholar]
- 37.Traupe H. The Ichthyoses: A Guide to Clinical Diagnostics, Genetic Counseling and Therapy. Heidelberg: Springer; 1989. [Google Scholar]
- 38.Anton-Lamprecht I. In: Diagnostic Ultrastructure of Non-neoplastic Diseases. Papadimitrou J M, Henderson D W, Spagnolo D V, editors. New York: Churchill Livingstone; 1992. pp. 459–551. [Google Scholar]
- 39.Jansen G A, Ofman R, Ferdinandusse S, Ijlst L, Muijsers A O, Skjeldal O H, Stokke O, Jakobs C, Besley G T, Wraith J E, et al. Nat Genet. 1997;17:190–193. doi: 10.1038/ng1097-190. [DOI] [PubMed] [Google Scholar]
- 40.De Laurenzi V, Rogers G R, Hamrock D J, Marekov L N, Steinert P M, Compton J G, Markova N G, Rizzo W B. Nat Genet. 1996;12:52–57. doi: 10.1038/ng0196-52. [DOI] [PubMed] [Google Scholar]
- 41.Russell L J, DiGiovanna J J, Rogers G R, Steinert P M, Hashem N, Compton J G, Bale S J. Nat Genet. 1995;9:279–283. doi: 10.1038/ng0395-279. [DOI] [PubMed] [Google Scholar]
- 42.Huber M, Rettler I, Bernasconi K, Frenk E, Lavrijsen S P, Ponec M, Bon A, Lautenschlager S, Schorderet D F, Hohl D. Science. 1995;267:525–528. doi: 10.1126/science.7824952. [DOI] [PubMed] [Google Scholar]
- 43.Matsuki M, Yamashita F, Ishida-Yamamoto A, Yamada K, Kinoshita C, Fushiki S, Ueda E, Morishima Y, Tabata K, Yasuno H, et al. Proc Natl Acad Sci USA. 1998;95:1044–1049. doi: 10.1073/pnas.95.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams M L. Semin Dermatol. 1992;11:169–175. [PubMed] [Google Scholar]
- 45.Hammarstroem S. J Lipid Res. 1971;12:760–765. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.