Full Text
The Full Text of this article is available as a PDF (19.8 MB).
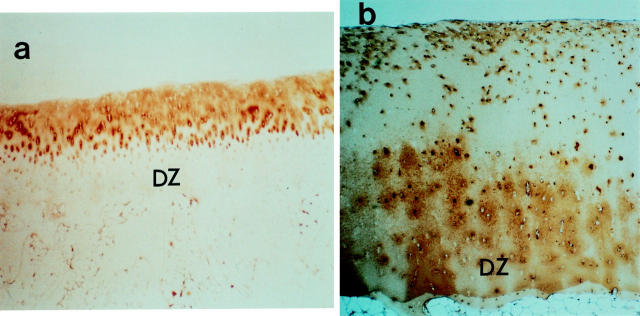
Figure 1 .
Immunohistochemical identification of type II collagen degradation in rheumatoid articular cartilage. Tibial plateau articular cartilage (woman 52 years) taken apart from joint margins were fixed for six hours at 4°C in 2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate solution, and washed at 4°C with 0.01 M phosphate buffer saline (PBS, pH 7.2) containing glycerol, as previously described by McLean and Nakano.19 Then they were decalcified with EDTA-glycerol solution at −5°C. The samples were embedded in paraffin wax, and immunohistochemical analysis was assessed on the sections using the avidin-biotin-peroxidase complex (ABC) immunoperoxidase.20 The cartilage sections were stained with monoclonal antibody against human type II collagen (a) and rat polyclonal antibody against CNBr derived peptides of type II collagen (b). Less staining for type II collagen and intense staining for CNBr derived peptides of type II collagen in the deep zone (DZ) matrix are observed. ((a) Original magnification × 6.6, (b) original magnification × 13.2).
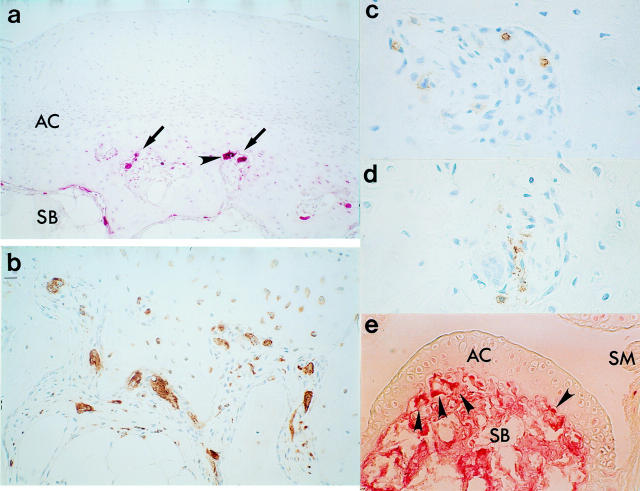
Figure 2 .
Immunostaining of sample tissues from RA patients (a-d) and MRL/Mp-lpr/lpr mouse (e). The staining for TRAP was performed according to the method of Burstone.21 Immunostaining was done as described in figure 1, using mouse monoclonal antibody against human CD68 (Dako, Denmark), HLA-DR (Dako, Denmark), and leucocyte T cell (MT1, Bio-science products, Switzerland). (a) Islands (arrows) that invaded into the deep zone of articular cartilage (AC) through the calcified cartilage from the subchondral bone (SB). TRAP positive multinucleated cells (arrowhead); (b) CD68 positive cells; (c) MT-1 positive cells; (d) HLA-DR positive cells in islands and subchondral bone. (e) TRAP positive multinucleated cells (arrows) beneath undamaged AC preceding remarkable inflammation of the synovial membrane (SM). ((a) Original magnification × 13.2, (b) original magnification × 66, (c) to (e) original magnification × 132).
Figure 3 .
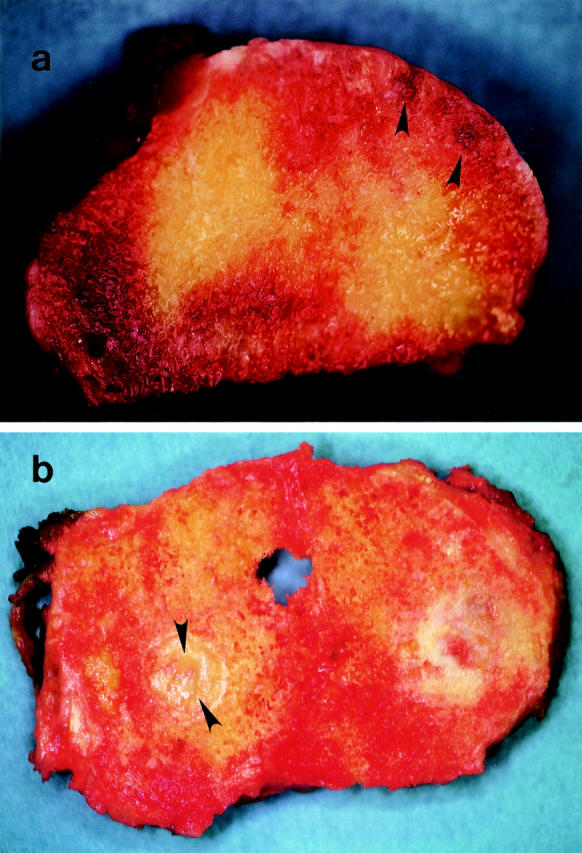
Macroscopical photograph of slices taken through the femoral head (a frontal section) and tibial plateau (a horizontal section) removed from 27 year old female and 56 year old female RA patients, respectively. (a) Small cystic areas are observed in the subchondral bone of the superior surface of a femoral head. (b) An area of cystic change in subchondral bone of medial tibial plateau. Such cysts (a, b) are seen apart from bare area and in the existence of the overlying articular cartilage (b, cartilage is opposite side of the cyst in the photograph).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURSTONE M. S. Histochemical demonstration of acid phosphatases with naphthol AS-phosphates. J Natl Cancer Inst. 1958 Sep;21(3):523–539. [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocco U., Cozzi L., Rigon C., Chieco-Bianchi F., Baldovin M., Cassisi G. A., Gallo C., Doria A., Favaro M. A., Piccoli A. Arthroscopic synovectomy in rheumatoid and psoriatic knee joint synovitis: long-term outcome. Br J Rheumatol. 1996 May;35(5):463–470. doi: 10.1093/rheumatology/35.5.463. [DOI] [PubMed] [Google Scholar]
- Fujii K., Tsuji M., Kitamura A., Murota K. The diagnostic significance of anti-type II collagen antibody assay in rheumatoid arthritis. Int Orthop. 1992;16(3):272–276. doi: 10.1007/BF00182710. [DOI] [PubMed] [Google Scholar]
- Fujii K., Tsuji M., Murota K., Terato K., Shimozuru Y., Nagai Y. An improved enzyme-linked immunosorbent assay of anti-collagen antibodies in human serum. J Immunol Methods. 1989 Nov 13;124(1):63–70. doi: 10.1016/0022-1759(89)90186-5. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Koopman W. J., Gay S. The MRL-lpr/lpr mouse. A model for the study of rheumatoid arthritis. Scand J Rheumatol Suppl. 1988;75:284–289. doi: 10.3109/03009748809096780. [DOI] [PubMed] [Google Scholar]
- Laskin R. S. Total condylar knee replacement in patients who have rheumatoid arthritis. A ten-year follow-up study. J Bone Joint Surg Am. 1990 Apr;72(4):529–535. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Riches D. W., Stanworth D. R. Evidence for a mechanism for the initiation of acid hydrolase secretion by macrophages that is functionally independent of alternative pathway complement activation. Biochem J. 1982 Mar 15;202(3):639–645. doi: 10.1042/bj2020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki N., Sudo Y., Yamane A., Sugihara S., Takai Y., Ishihara K., Ono S., Hamaoka T., Senoh H., Fujiwara H. Type II collagen-induced murine arthritis. II. Genetic control of arthritis induction is expressed on L3T4+ T cells required for humoral as well as cell-mediated immune responses to type II collagen. Reg Immunol. 1989 Jul-Aug;2(4):203–212. [PubMed] [Google Scholar]
- Snowden N., Kay R. A. Immunology of systemic rheumatoid disease. Br Med Bull. 1995 Apr;51(2):437–448. doi: 10.1093/oxfordjournals.bmb.a072971. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Dixon F. J. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983 Aug 1;158(2):378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A., Holmdahl R., Rubin K., Klareskog L., Nilsson L. A., Gunnarsson K. Patterns of autoreactivity to collagen type II in autoimmune MRL/l mice. Clin Exp Immunol. 1986 Feb;63(2):441–449. [PMC free article] [PubMed] [Google Scholar]
- Terato K., Shimozuru Y., Katayama K., Takemitsu Y., Yamashita I., Miyatsu M., Fujii K., Sagara M., Kobayashi S., Goto M. Specificity of antibodies to type II collagen in rheumatoid arthritis. Arthritis Rheum. 1990 Oct;33(10):1493–1500. doi: 10.1002/art.1780331006. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]