Abstract
OBJECTIVE—Wegener's granulomatosis (WG) is an inflammatory disorder characterised by granulomatous inflammation, vasculitis, and necrotising vasculitis and is strongly associated with anti-neutrophil cytoplasmic antibodies (ANCA). Activated monocytes/macrophages are present in renal biopsy specimens and participate in granuloma formation by synthesising and secreting a variety of chemoattractants, growth factors, and cytokines. In view of these findings, in vivo monocyte activation was evaluated in patients with WG and the findings related to parameters of clinical disease activity. METHODS—Monocyte activation was analysed by measuring plasma concentrations of soluble products of monocyte activation, that is neopterin and interleukin 6 (IL6), by ELISA, and by quantitating the surface expression of activation markers on circulating monocytes by flow cytometry. RESULTS—Twenty four patients with active WG were included in this study. Ten of these patients were also analysed at the time of remission. Twelve patients with sepsis served as positive controls, and 10 healthy volunteers as negative controls for monocyte activation. Patients with active disease had increased monocyte activation compared with healthy controls as shown by increased concentrations of neopterin (p <0.0001) and increased surface expression of CD11b (p < 0.05) and CD64 (p < 0.05). In those patients with increased concentrations of IL6 during active disease plasma concentrations of IL6 decreased during follow up when patients went into remission (p < 0.0001). In addition, neopterin (r = 0.37, r = 0.44), IL6 (r = 0.37, r = 0.60) and CD63 expression (r = 0.39, r = 0.45) correlated significantly with disease activity as measured by the Birmingham Vasculitis Activity Score and C reactive protein values, respectively. Compared with patients with sepsis, all markers of monocyte activation in patients with vasculitis were lower. CONCLUSION—It is concluded that disease activity in WG correlates with the extent of activation of monocytes, compatible with their role in the pathophysiology of this disease. Keywords: anti-neutrophil cytoplasmic antibody; monocyte activation; vasculitis; flow cytometry
Full Text
The Full Text of this article is available as a PDF (166.2 KB).
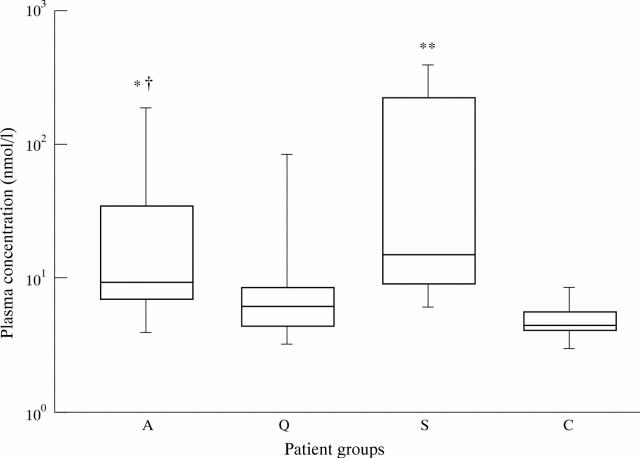
Figure 1 .
Box and whisker plots indicating the overall range (error bars), 25-75% range (boxes), and median value (horizontal lines) of neopterin plasma concentrations in patients with WG (A: active disease, Q: quiescent disease) compared with concentrations in patients with sepsis (S) and healthy controls (C). * p < 0.05, ** p < 0.0001 compared with healthy controls, † p = 0.074 compared with quiescent disease.
Figure 2 .
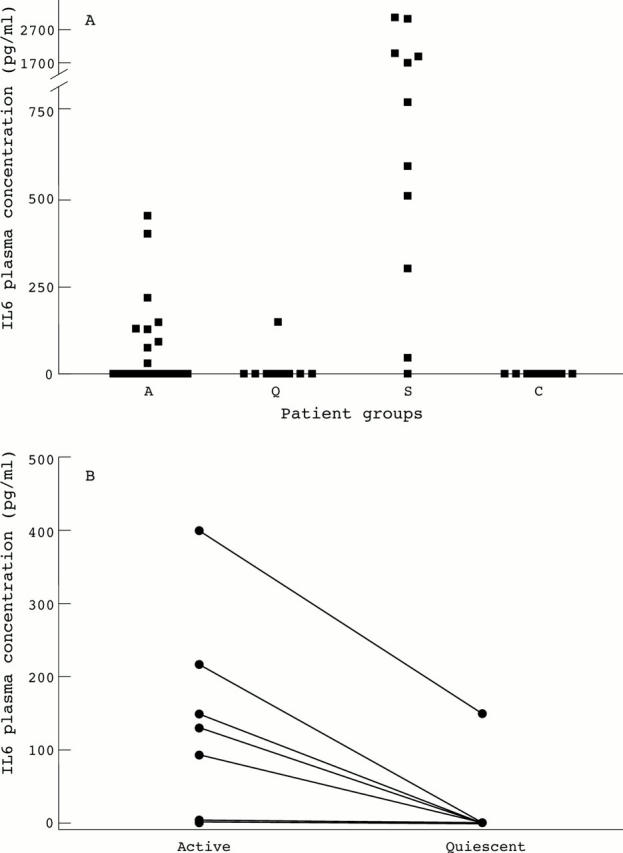
(A) A dot blot of the plasma concentrations of IL6 in 24 patients with WG (A: active disease, open squares represent 12 patients with newly diagnosed disease, closed squares represent 12 patients with relapsing disease, Q: quiescent disease, 10 patients) compared with concentrations in 12 patients with sepsis (S) and healthy controls (C). (B) Paired observations of IL6 plasma concentrations in 10 patients with active and quiescent disease. p<0.0001.
Figure 3 .
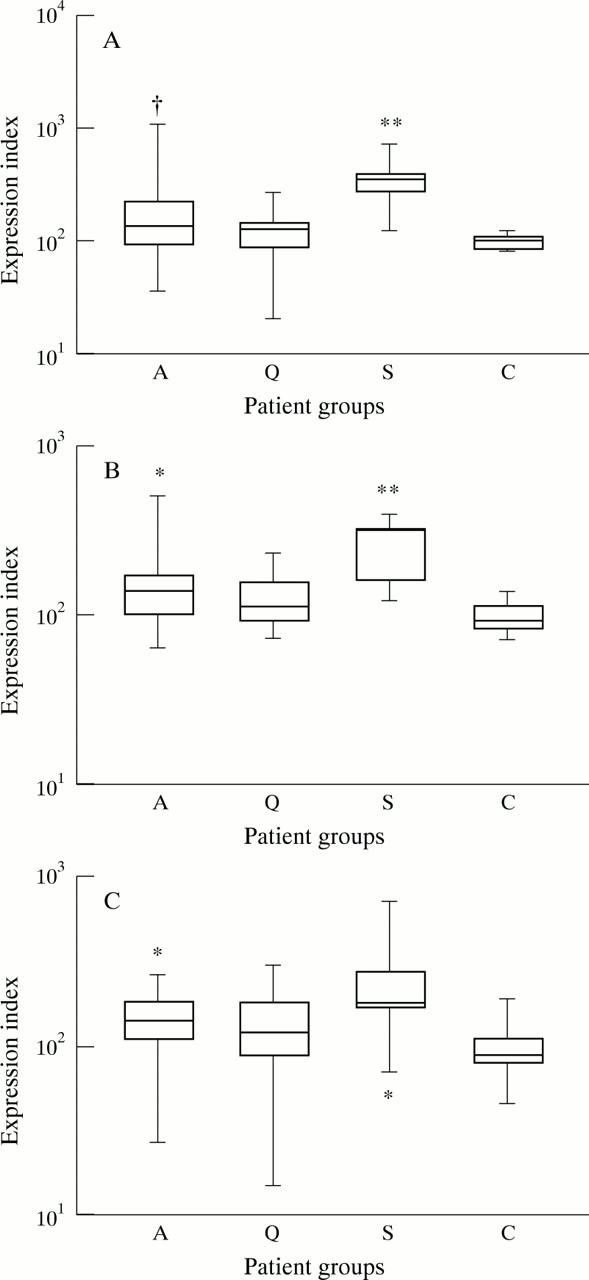
(A) Box and whisker plots of the surface expression CD63 on monocytes from patients with WG (A: active disease, Q: quiescent disease) compared with the expression on cells from patients with sepsis (S) and healthy controls (C). † p = 0.0787, ** p < 0.0001 compared with healthy controls. (B) Box and whisker plots of the surface expression of CD11b on monocytes from patients with WG (A: active disease, Q: quiescent disease) compared with the expression on cells from patients with sepsis (S) and healthy controls (C). * p < 0.05, ** p < 0.0001 compared with healthy controls. (C) Box and whisker plots of the surface expression of CD64 on monocytes from patients with WG (A: active disease, Q: quiescent disease) compared with the expression on cells from patients with sepsis (S) and healthy controls (C). * p < 0.05 compared with healthy controls.
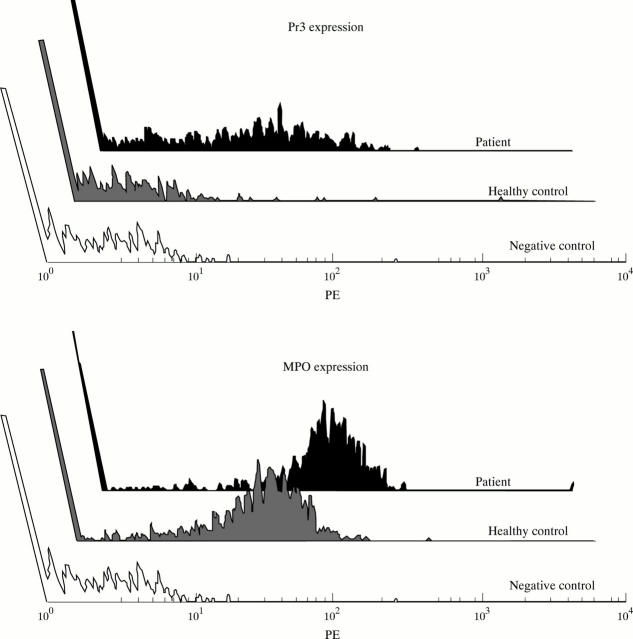
Figure 4 .
Flow cytometry histogram representing membrane expression of Pr3 and MPO and the negative control staining on monocytes from a patient with active WG and a healthy control. Cell count and the mean fluorescence intensity (MFI) are depicted on the y and x axes respectively.
Figure 5 .
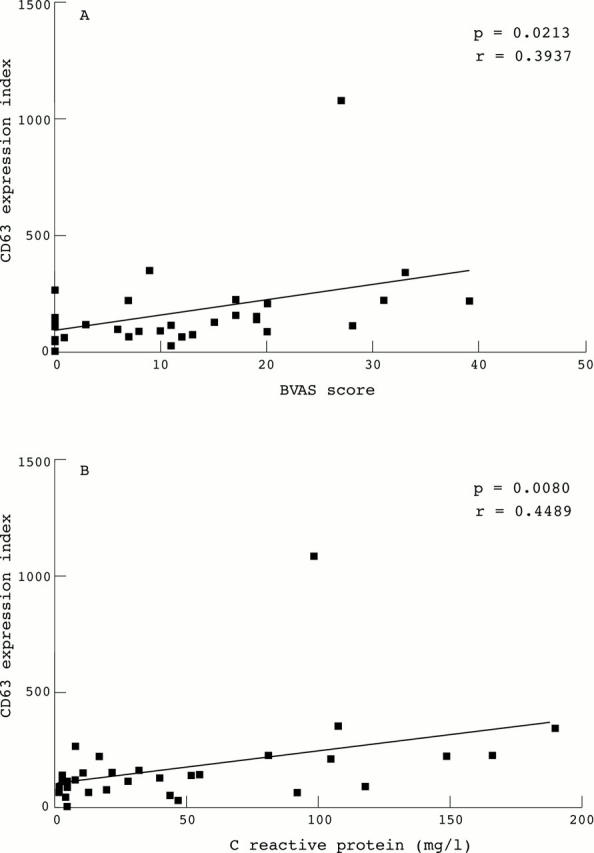
Surface expression of CD63 on monocytes (expressed as expression index, see methods) correlate with disease activity as expressed by the BVAS score (A) or CRP values (B).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulitzky W. E., Tilg H., Niederwieser D., Riccabona G., Obendorf L., Margreiter R., Pfaller W., Huber C. Comparison of serum neopterin levels and urinary neopterin excretion in renal allograft recipients. Clin Nephrol. 1988 May;29(5):248–252. [PubMed] [Google Scholar]
- Casselman B. L., Kilgore K. S., Miller B. F., Warren J. S. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J Lab Clin Med. 1995 Nov;126(5):495–502. [PubMed] [Google Scholar]
- Charles L. A., Caldas M. L., Falk R. J., Terrell R. S., Jennette J. C. Antibodies against granule proteins activate neutrophils in vitro. J Leukoc Biol. 1991 Dec;50(6):539–546. doi: 10.1002/jlb.50.6.539. [DOI] [PubMed] [Google Scholar]
- Csernok E., Ernst M., Schmitt W., Bainton D. F., Gross W. L. Activated neutrophils express proteinase 3 on their plasma membrane in vitro and in vivo. Clin Exp Immunol. 1994 Feb;95(2):244–250. doi: 10.1111/j.1365-2249.1994.tb06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert B. H., Becker M. E., Jennette J. C., Falk R. J. Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail. 1995 Mar;17(2):125–133. doi: 10.3109/08860229509026249. [DOI] [PubMed] [Google Scholar]
- Exley A. R., Bacon P. A., Luqmani R. A., Kitas G. D., Gordon C., Savage C. O., Adu D. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997 Feb;40(2):371–380. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Hogan S., Carey T. S., Jennette J. C. Clinical course of anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and systemic vasculitis. The Glomerular Disease Collaborative Network. Ann Intern Med. 1990 Nov 1;113(9):656–663. doi: 10.7326/0003-4819-113-9-656. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Terrell R. S., Charles L. A., Jennette J. C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Haynes B. F., Katz P., Wolff S. M. Wegener's granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983 Jan;98(1):76–85. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- Fuchs D., Weiss G., Reibnegger G., Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci. 1992;29(3-4):307–341. doi: 10.3109/10408369209114604. [DOI] [PubMed] [Google Scholar]
- Haller H., Eichhorn J., Pieper K., Göbel U., Luft F. C. Circulating leukocyte integrin expression in Wegener's granulomatosis. J Am Soc Nephrol. 1996 Jan;7(1):40–48. doi: 10.1681/ASN.V7140. [DOI] [PubMed] [Google Scholar]
- Haupt W., Hohenberger W., Klein P., Christou N. V. Detection of neopterin, interleukin-6 and acute-phase proteins as parameters of potential monocyte activation in preoperative patients. Infection. 1995 Sep-Oct;23(5):263–266. doi: 10.1007/BF01716282. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Horneff G., Sack U., Kalden J. R., Emmrich F., Burmester G. R. Reduction of monocyte-macrophage activation markers upon anti-CD4 treatment. Decreased levels of IL-1, IL-6, neopterin and soluble CD14 in patients with rheumatoid arthritis. Clin Exp Immunol. 1993 Feb;91(2):207–213. doi: 10.1111/j.1365-2249.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenberg C. G., Brouwer E., Weening J. J., Tervaert J. W. Anti-neutrophil cytoplasmic antibodies: current diagnostic and pathophysiological potential. Kidney Int. 1994 Jul;46(1):1–15. doi: 10.1038/ki.1994.239. [DOI] [PubMed] [Google Scholar]
- Kuijpers T. W., Tool A. T., van der Schoot C. E., Ginsel L. A., Onderwater J. J., Roos D., Verhoeven A. J. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991 Aug 15;78(4):1105–1111. [PubMed] [Google Scholar]
- Leavitt R. Y., Fauci A. S., Bloch D. A., Michel B. A., Hunder G. G., Arend W. P., Calabrese L. H., Fries J. F., Lie J. T., Lightfoot R. W., Jr The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990 Aug;33(8):1101–1107. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- Lioté F., Boval-Boizard B., Weill D., Kuntz D., Wautier J. L. Blood monocyte activation in rheumatoid arthritis: increased monocyte adhesiveness, integrin expression, and cytokine release. Clin Exp Immunol. 1996 Oct;106(1):13–19. doi: 10.1046/j.1365-2249.1996.d01-820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqmani R. A., Bacon P. A., Moots R. J., Janssen B. A., Pall A., Emery P., Savage C., Adu D. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM. 1994 Nov;87(11):671–678. [PubMed] [Google Scholar]
- Mayet W. J., Meyer zum Büschenfelde K. H. Antibodies to proteinase 3 increase adhesion of neutrophils to human endothelial cells. Clin Exp Immunol. 1993 Dec;94(3):440–446. doi: 10.1111/j.1365-2249.1993.tb08215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzelaar M. J., Wijngaard P. L., Peters P. J., Sixma J. J., Nieuwenhuis H. K., Clevers H. C. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991 Feb 15;266(5):3239–3245. [PubMed] [Google Scholar]
- Mulder A. H., Broekroelofs J., Horst G., Limburg P. C., Nelis G. F., Kallenberg C. G. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease: characterization and clinical correlates. Clin Exp Immunol. 1994 Mar;95(3):490–497. doi: 10.1111/j.1365-2249.1994.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder A. H., Heeringa P., Brouwer E., Limburg P. C., Kallenberg C. G. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a Fc gamma RII-dependent process. Clin Exp Immunol. 1994 Nov;98(2):270–278. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller Kobold A. C., Kallenberg C. G., Tervaert J. W. Leucocyte membrane expression of proteinase 3 correlates with disease activity in patients with Wegener's granulomatosis. Br J Rheumatol. 1998 Aug;37(8):901–907. doi: 10.1093/rheumatology/37.8.901. [DOI] [PubMed] [Google Scholar]
- Nassonov E., Samsonov M., Beketova T., Semenkova L., Wachter H., Fuchs D. Serum neopterin concentrations in Wegener's granulomatosis correlate with vasculitis activity. Clin Exp Rheumatol. 1995 May-Jun;13(3):353–356. [PubMed] [Google Scholar]
- Pinching A. J., Rees A. J., Pussell B. A., Lockwood C. M., Mitchison R. S., Peters D. K. Relapses in Wegener's granulomatosis: the role of infection. Br Med J. 1980 Sep 27;281(6244):836–838. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges A. J., Redecha P. B., Kimberly W. T., Csernok E., Gross W. L., Kimberly R. P. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994 Aug 1;153(3):1271–1280. [PubMed] [Google Scholar]
- Ralston D. R., Marsh C. B., Lowe M. P., Wewers M. D. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997 Sep 15;100(6):1416–1424. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastaldi M. P., Ferrario F., Tunesi S., Yang L., D'Amico G. Intraglomerular and interstitial leukocyte infiltration, adhesion molecules, and interleukin-1 alpha expression in 15 cases of antineutrophil cytoplasmic autoantibody-associated renal vasculitis. Am J Kidney Dis. 1996 Jan;27(1):48–57. doi: 10.1016/s0272-6386(96)90030-x. [DOI] [PubMed] [Google Scholar]
- Riecken B., Gutfleisch J., Schlesier M., Peter H. H. Impaired granulocyte oxidative burst and decreased expression of leucocyte adhesion molecule-1 (LAM-1) in patients with Wegener's granulomatosis. Clin Exp Immunol. 1994 Apr;96(1):43–47. doi: 10.1111/j.1365-2249.1994.tb06227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonov M. Y., Tilz G. P., Egorova O., Reibnegger G., Balabanova R. M., Nassonov E. L., Nassonova V. A., Wachter H., Fuchs D. Serum soluble markers of immune activation and disease activity in systemic lupus erythematosus. Lupus. 1995 Feb;4(1):29–32. doi: 10.1177/096120339500400107. [DOI] [PubMed] [Google Scholar]
- Savage C. O., Pottinger B. E., Gaskin G., Pusey C. D., Pearson J. D. Autoantibodies developing to myeloperoxidase and proteinase 3 in systemic vasculitis stimulate neutrophil cytotoxicity toward cultured endothelial cells. Am J Pathol. 1992 Aug;141(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Jacobberger J. W. Flow cytometric titration of retroviral expression vectors: comparison of methods for analysis of immunofluorescence histograms derived from cells expressing low antigen levels. Cytometry. 1993;14(1):23–31. doi: 10.1002/cyto.990140106. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stegeman C. A., Tervaert J. W., Huitema M. G., de Jong P. E., Kallenberg C. G. Serum levels of soluble adhesion molecules intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and E-selectin in patients with Wegener's granulomatosis. Relationship to disease activity and relevance during followup. Arthritis Rheum. 1994 Aug;37(8):1228–1235. doi: 10.1002/art.1780370818. [DOI] [PubMed] [Google Scholar]
- Stegeman C. A., Tervaert J. W., Sluiter W. J., Manson W. L., de Jong P. E., Kallenberg C. G. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994 Jan 1;120(1):12–17. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- Stegeman C. A., Tervaert J. W., de Jong P. E., Kallenberg C. G. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996 Jul 4;335(1):16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Goldschmeding R., Elema J. D., van der Giessen M., Huitema M. G., van der Hem G. K., The T. H., von dem Borne A. E., Kallenberg C. G. Autoantibodies against myeloid lysosomal enzymes in crescentic glomerulonephritis. Kidney Int. 1990 Feb;37(2):799–806. doi: 10.1038/ki.1990.48. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Huitema M. G., Hené R. J., Sluiter W. J., The T. H., van der Hem G. K., Kallenberg C. G. Prevention of relapses in Wegener's granulomatosis by treatment based on antineutrophil cytoplasmic antibody titre. Lancet. 1990 Sep 22;336(8717):709–711. doi: 10.1016/0140-6736(90)92205-v. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Kallenberg C. G. Cell adhesion molecules in vasculitis. Curr Opin Rheumatol. 1997 Jan;9(1):16–25. doi: 10.1097/00002281-199701000-00004. [DOI] [PubMed] [Google Scholar]
- Tervaert J. W., Mulder L., Stegeman C., Elema J., Huitema M., The H., Kallenberg C. Occurrence of autoantibodies to human leucocyte elastase in Wegener's granulomatosis and other inflammatory disorders. Ann Rheum Dis. 1993 Feb;52(2):115–120. doi: 10.1136/ard.52.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerius T., Repp R., de Wit T. P., Berthold S., Platzer E., Kalden J. R., Gramatzki M., van de Winkel J. G. Involvement of the high-affinity receptor for IgG (Fc gamma RI; CD64) in enhanced tumor cell cytotoxicity of neutrophils during granulocyte colony-stimulating factor therapy. Blood. 1993 Aug 1;82(3):931–939. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]