Abstract
OBJECTIVES—Chondrocytic matrix metalloproteinases (MMPs) are believed to be important in osteoarthritic cartilage degradation. The cartilage lesion of osteoarthritis (OA) is focal and often progressive. During its development chondrocytes differentially up and down regulate production of mRNA for individual MMPs. This observation has potential implications for understanding the disease processes that lead to progressive cartilage loss in OA and designing appropriate targeted treatment. The complex regulation of MMP mediated effects means there is a pressing need to establish whether visualisation of MMP mRNA or protein equates to enzyme activity. The technique of in situ zymography (ISZ) offers a way of examining diseased human tissue for in vivo production of an excess of degrading enzyme over inhibitor. The primary objective of this study was to assess, and if positive follow, collagen II degrading activity in cartilage during development of the OA lesion. A secondary objective was to assess whether there was any correlation between sites of collagen II degrading activity and expression of the collagenase (MMP-13), recently implicated in type II collagen degredation in this lesion. METHODS—Biopsied human normal and osteoarthritic cartilage, showing various degrees of damage, was examined by in situ zymography, with and without enzyme inhibitors, to establish sites of type II collagenase activity. Paired samples were probed for MMP-13 mRNA using 35S-labelled oligonucleotide probes. Comparative analyses were performed. RESULTS—In situ zymography showed collagen II degrading activity over chondrocytes only in osteoarthritic cartilage. Distribution and amount varied with the extent of cartilage damage and position of chondrocytes, being greatest in deep cartilage and in cartilage lesions where fissuring was occurring. The enzyme causing the degradation behaved as a matrix metalloproteinase. MMP-13 mRNA expression codistributed with the type II collagenase activity. CONCLUSION—In OA, chondrocytes can degrade type II collagen. The type II collagen degrading activity varies in site and amount as the cartilage lesion progresses and throughout codistributes with MMP-13 mRNA expression.
Full Text
The Full Text of this article is available as a PDF (5.1 MB).
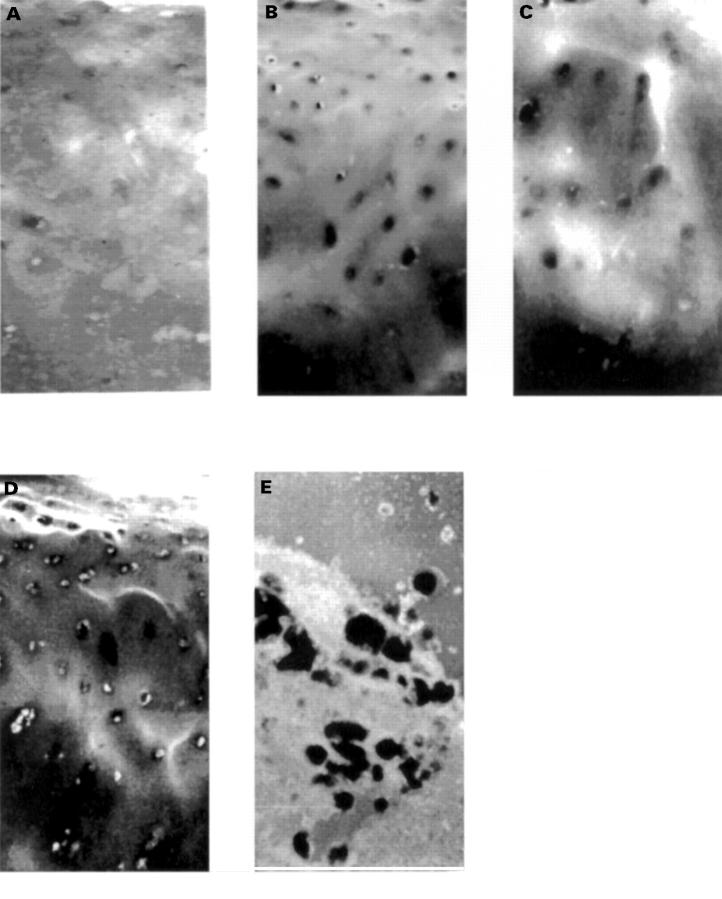
Figure 1 .
Sections of articular surface reacted by radioactive ISH for MMP13 mRNA. (A)-(D) The cartilage surface is at the top. The darker staining area at the bottom is calcified cartilage (zone 4). The four sets of figures are grade 0, 1, 2, 3 lesions (A to D respectively). The figure on the left is probed with antisense oligonucleotides and that on the right with sense probes. Hybridisation has been demonstrated by autoradiography, and is seen as black grains over the cells. These slides have been exposed for 70 days so that the distribution could be demonstrated at low magnification (×10) to show spatial variation and relation to cartilage damage in each grade. (E) Both the photomicrographs in (E) are examples of the reaction product at 35 days viewed by dark field illumination. This is the length of exposure used for the measurements given in table 1. Magnification × 100.
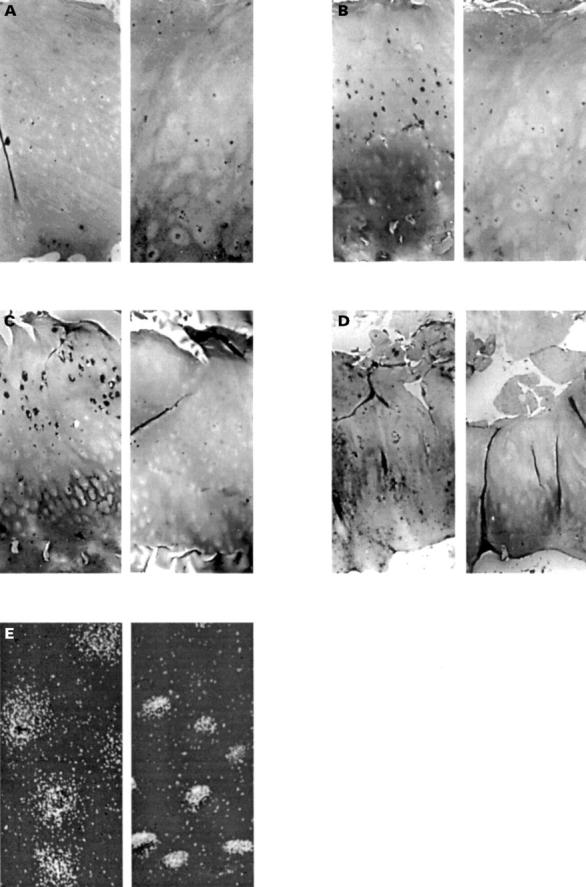
Figure 2 .
Representative figures of the collagenase II ISZ to illustrate the appearances of the ISZ reaction. All the figures are from the same grade 1 cartilage specimen. (A) is time 0 and (B) is 48 hours with no inhibitors. (C) is a serial section reacted with MSI. It shows decreased reaction area. (D) is a similar area to (B) viewed in dark field Nomarski optics and shows the nuclei of the chondrocytes particularly well. All images taken at × 100 magnification. (E) is the positive control, rheumatoid synovium, also at 48 hours. Note the areas of gel digestion below regions of the subintima and a villous surface projection × 250.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billinghurst R. C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., Mitchell P., Hambor J., Diekmann O., Tschesche H. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997 Apr 1;99(7):1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunning R. A., Richardson H. J., Crawford A., Skjodt H., Hughes D., Evans D. B., Gowen M., Dobson P. R., Brown B. L., Russell R. G. The effect of interleukin-1 on connective tissue metabolism and its relevance to arthritis. Agents Actions Suppl. 1986;18:131–152. doi: 10.1007/978-3-0348-7684-1_19. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Bramwell H., Hollander A. P. Proteolytic mechanisms of cartilage breakdown: a target for arthritis therapy? Clin Mol Pathol. 1995 Aug;48(4):M167–M177. doi: 10.1136/mp.48.4.m167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case J. P., Lafyatis R., Remmers E. F., Kumkumian G. K., Wilder R. L. Transin/stromelysin expression in rheumatoid synovium. A transformation-associated metalloproteinase secreted by phenotypically invasive synoviocytes. Am J Pathol. 1989 Dec;135(6):1055–1064. [PMC free article] [PubMed] [Google Scholar]
- Cawston T. E., Billington C. Metalloproteinases in the rheumatic diseases. J Pathol. 1996 Oct;180(2):115–117. doi: 10.1002/(SICI)1096-9896(199610)180:2<115::AID-PATH674>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Cazorla M., Hernández L., Nadal A., Balbín M., López J. M., Vizoso F., Fernández P. L., Iwata K., Cardesa A., López-Otín C. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol. 1998 Oct;186(2):144–150. doi: 10.1002/(SICI)1096-9896(1998100)186:2<144::AID-PATH147>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dean D. D., Azzo W., Martel-Pelletier J., Pelletier J. P., Woessner J. F., Jr Levels of metalloproteases and tissue inhibitor of metalloproteases in human osteoarthritic cartilage. J Rheumatol. 1987 May;14(Spec No):43–44. [PubMed] [Google Scholar]
- Dean D. D., Martel-Pelletier J., Pelletier J. P., Howell D. S., Woessner J. F., Jr Evidence for metalloproteinase and metalloproteinase inhibitor imbalance in human osteoarthritic cartilage. J Clin Invest. 1989 Aug;84(2):678–685. doi: 10.1172/JCI114215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemont A. J., Hampson V., Tilman R., Goupille P., Taiwo Y., Hoyland J. A. Gene expression of matrix metalloproteinases 1, 3, and 9 by chondrocytes in osteoarthritic human knee articular cartilage is zone and grade specific. Ann Rheum Dis. 1997 Sep;56(9):542–549. doi: 10.1136/ard.56.9.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994 Jun 17;269(24):16766–16773. [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Libby P. Microscopic localization of active proteases by in situ zymography: detection of matrix metalloproteinase activity in vascular tissue. FASEB J. 1995 Jul;9(10):974–980. doi: 10.1096/fasebj.9.10.7615167. [DOI] [PubMed] [Google Scholar]
- Hollander A. P., Pidoux I., Reiner A., Rorabeck C., Bourne R., Poole A. R. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995 Dec;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyland J. A., Freemont A. J. Investigation of a quantitative post-hybridization signal amplification system for mRNA-oligodeoxyribonucleotide in situ hybridization. J Pathol. 1991 May;164(1):51–58. doi: 10.1002/path.1711640110. [DOI] [PubMed] [Google Scholar]
- Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996 Jan 19;271(3):1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- Lefebvre V., Peeters-Joris C., Vaes G. Production of gelatin-degrading matrix metalloproteinases ('type IV collagenases') and inhibitors by articular chondrocytes during their dedifferentiation by serial subcultures and under stimulation by interleukin-1 and tumor necrosis factor alpha. Biochim Biophys Acta. 1991 Aug 13;1094(1):8–18. doi: 10.1016/0167-4889(91)90020-x. [DOI] [PubMed] [Google Scholar]
- Lindy O., Konttinen Y. T., Sorsa T., Ding Y., Santavirta S., Ceponis A., López-Otín C. Matrix metalloproteinase 13 (collagenase 3) in human rheumatoid synovium. Arthritis Rheum. 1997 Aug;40(8):1391–1399. doi: 10.1002/art.1780400806. [DOI] [PubMed] [Google Scholar]
- Marles P. J., Hoyland J. A., Parkinson R., Freemont A. J. Demonstration of variation in chondrocyte activity in different zones of articular cartilage: an assessment of the value of in-situ hybridization. Int J Exp Pathol. 1991 Apr;72(2):171–182. [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., Fujimoto N., Obata K., Cloutier J. M., Pelletier J. P. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994 Jun;70(6):807–815. [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P. Neutral proteases in human osteoarthritic synovium: quantification and characterization. J Rheumatol. 1987 May;14(Spec No):38–40. [PubMed] [Google Scholar]
- Martel-Pelletier J., Pelletier J. P. Wanted--the collagenase responsible for the destruction of the collagen network in human cartilage! Br J Rheumatol. 1996 Sep;35(9):818–820. doi: 10.1093/rheumatology/35.9.818. [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Zafarullah M., Kodama S., Pelletier J. P. In vitro effects of interleukin 1 on the synthesis of metalloproteases, TIMP, plasminogen activators and inhibitors in human articular cartilage. J Rheumatol Suppl. 1991 Feb;27:80–84. [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- Mehraban F., Kuo S. Y., Riera H., Chang C., Moskowitz R. W. Prostromelysin and procollagenase genes are differentially up-regulated in chondrocytes from the knees of rabbits with experimental osteoarthritis. Arthritis Rheum. 1994 Aug;37(8):1189–1197. doi: 10.1002/art.1780370813. [DOI] [PubMed] [Google Scholar]
- Mitchell P. G., Magna H. A., Reeves L. M., Lopresti-Morrow L. L., Yocum S. A., Rosner P. J., Geoghegan K. F., Hambor J. E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996 Feb 1;97(3):761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan F., Pelletier J. P., Hambor J., Cloutier J. M., Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997 Sep;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Dodge G. R., Roughley P. J., Liu J., Finch S. J., DiPasquale G., Poole A. R. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix. 1993 Mar;13(2):95–102. doi: 10.1016/s0934-8832(11)80068-5. [DOI] [PubMed] [Google Scholar]
- Panula H. E., Hyttinen M. M., Arokoski J. P., Långsjö T. K., Pelttari A., Kiviranta I., Helminen H. J. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis. 1998 Apr;57(4):237–245. doi: 10.1136/ard.57.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J. P., Martel-Pelletier J., Malemud C. J. Proteoglycans from experimental osteoarthritic cartilage: degradation by neutral metalloproteases. J Rheumatol. 1987 May;14(Spec No):113–115. [PubMed] [Google Scholar]
- Pelletier J. P., Mineau F., Faure M. P., Martel-Pelletier J. Imbalance between the mechanisms of activation and inhibition of metalloproteases in the early lesions of experimental osteoarthritis. Arthritis Rheum. 1990 Oct;33(10):1466–1476. doi: 10.1002/art.1780331003. [DOI] [PubMed] [Google Scholar]
- Pelletier J. P., Roughley P. J., DiBattista J. A., McCollum R., Martel-Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin Arthritis Rheum. 1991 Jun;20(6 Suppl 2):12–25. doi: 10.1016/0049-0172(91)90024-t. [DOI] [PubMed] [Google Scholar]
- Reboul P., Pelletier J. P., Tardif G., Cloutier J. M., Martel-Pelletier J. The new collagenase, collagenase-3, is expressed and synthesized by human chondrocytes but not by synoviocytes. A role in osteoarthritis. J Clin Invest. 1996 May 1;97(9):2011–2019. doi: 10.1172/JCI118636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky S., Malemud C., Sheff M. The proteoglycanase from human cartilage and cultured rabbit chondrocytes and its relation to osteoarthritis. J Rheumatol. 1987 May;14(Spec No):33–35. [PubMed] [Google Scholar]
- Shingu M., Nagai Y., Isayama T., Naono T., Nobunaga M., Nagai Y. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993 Oct;94(1):145–149. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlopov B. V., Lie W. R., Mainardi C. L., Cole A. A., Chubinskaya S., Hasty K. A. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997 Nov;40(11):2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- Ståhle-Bäckdahl M., Sandstedt B., Bruce K., Lindahl A., Jiménez M. G., Vega J. A., López-Otín C. Collagenase-3 (MMP-13) is expressed during human fetal ossification and re-expressed in postnatal bone remodeling and in rheumatoid arthritis. Lab Invest. 1997 May;76(5):717–728. [PubMed] [Google Scholar]
- Vankemmelbeke M., Dekeyser P. M., Hollander A. P., Buttle D. J., Demeester J. Characterization of helical cleavages in type II collagen generated by matrixins. Biochem J. 1998 Mar 1;330(Pt 2):633–640. doi: 10.1042/bj3300633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L., Freemont A. J., Hoyland J. A. The effect of tissue decalcification on mRNA retention within bone for in-situ hybridization studies. Int J Exp Pathol. 1993 Jun;74(3):237–241. [PMC free article] [PubMed] [Google Scholar]
- Wernicke D., Seyfert C., Hinzmann B., Gromnica-Ihle E. Cloning of collagenase 3 from the synovial membrane and its expression in rheumatoid arthritis and osteoarthritis. J Rheumatol. 1996 Apr;23(4):590–595. [PubMed] [Google Scholar]
- Woessner J. F., Jr, Gunja-Smith Z. Role of metalloproteinases in human osteoarthritis. J Rheumatol Suppl. 1991 Feb;27:99–101. [PubMed] [Google Scholar]
- Wolfe G. C., MacNaul K. L., Buechel F. F., McDonnell J., Hoerrner L. A., Lark M. W., Moore V. L., Hutchinson N. I. Differential in vivo expression of collagenase messenger RNA in synovium and cartilage. Quantitative comparison with stromelysin messenger RNA levels in human rheumatoid arthritis and osteoarthritis patients and in two animal models of acute inflammatory arthritis. Arthritis Rheum. 1993 Nov;36(11):1540–1547. doi: 10.1002/art.1780361108. [DOI] [PubMed] [Google Scholar]