Abstract
OBJECTIVES—CD4+ T cells sustain the chronic synovial inflammatory response in rheumatoid arthritis (RA). SB-210396/CE 9.1 is an anti-CD4 monoclonal antibody that has documented efficacy in RA when given intravenously. This study aimed to establish the safety and efficacy of the intra-articular administration of SB-210396/CE 9.1 compared with placebo, examining its mode of action using a combined imaging approach of arthroscopy, magnetic resonance imaging (MRI), and histology. METHODS—Thirteen RA patients with active, resistant knee synovitis, were randomised to intra-articular injection of placebo (n=3), 0.4 mg (n=3) or 40 mg (n=7) of anti-CD4 after sequential dynamic gadolinium enhanced MRI, followed by same day arthroscopy and synovial membrane biopsy. Imaging and arthroscopic synovial membrane sampling were repeated at six weeks. This study used a unique region of interest (ROI) analysis mapping the MRI area analysed to the specific biopsy site identified arthroscopically, thus providing data for all three modalities at the same synovial membrane site. RESULTS—12 patients completed the study (one placebo treated patient refused further MRI). Arthroscopic improvement was observed in 0 of 2 placebo patients but in 10 of 10 patients receiving active drug (>20% in 6 of 10). Improvement in MRI was consistently observed in all patients of the 40 mg group but not in the other two groups. A reduction in SM CD4+ score was noted in the 40 mg group and in the 0.4 mg group. Strong correlations both before and after treatment, were identified between the three imaging modalities. Intra-articular delivery of SB-210396/CE 9.1 was well tolerated. CONCLUSIONS—SB-210396/CE 9.1 is safe when administered by intra-articular injection. A trend toward efficacy was found by coordinated MRI, arthroscopic, and histological imaging, not seen in the placebo group. The value of ROI analysis was demonstrated.
Full Text
The Full Text of this article is available as a PDF (18.0 MB).
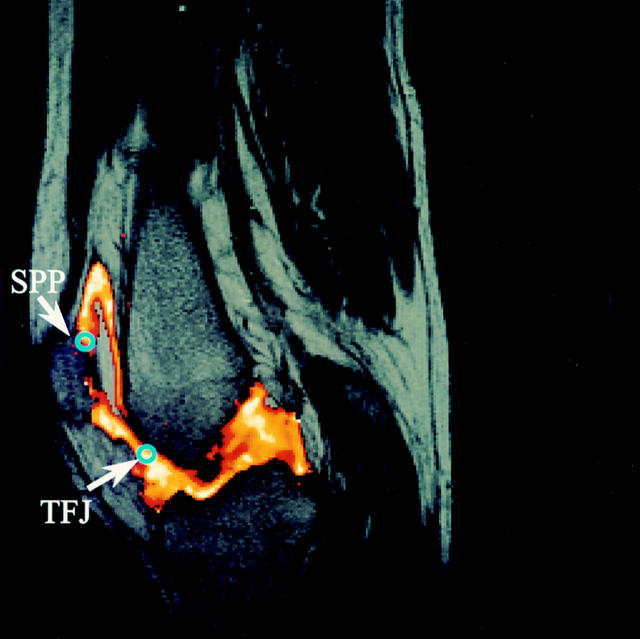
Figure 1 .
Magnetic resonance image of the knee joint. This demonstrates a typical dynamic T1 weighted gradient echo magnetic resonance image (TR/TE/flip angle 30/12/60° ) used to map with the computer localised regions of interest (area=11.8 mm2) at the suprapatellar pouch (SPP) and the tibiofemoral joint (TFJ).
Figure 2 .
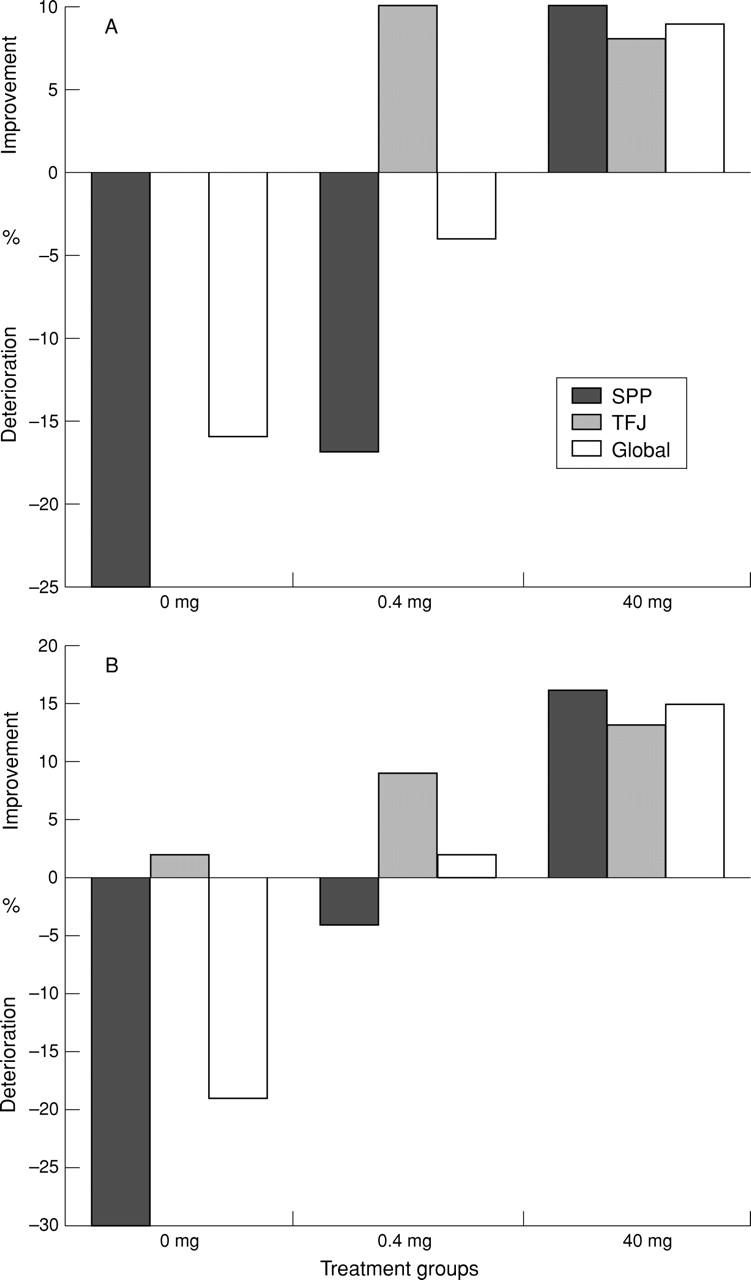
The percentage change of median dynamic enhanced (Gadolinium-DTPA) magnetic resonance imaging parameters over baseline with respect to: (A) maximum rate of normalised signal intensity enhancement (MRE) reflecting synovial capillary permeability; and (B) the maximum normalised signal intensity enhancement (ME) reflecting the perfusion and the volume of the extracellular fluid at the regions of interest and globally in the three treatment groups. (A) Deterioration in MRE is seen at both regions and globally in the placebo group. Ten per cent improvement is seen only at the tibiofemoral joint region in the 0.4 mg group. Improvement (up to 10%) is seen at both regions of interest and globally in the group treated with 40 mg of active drug. (B) Deterioration is seen at the suprapatellar region and globally in the placebo group. Mild improvement (<10%) is seen at the tibiofemoral joint region and globally in the 0.4 mg group. Improvement (10-20%) is seen at both regions of interest and globally in the group treated with 40 mg of active drug.
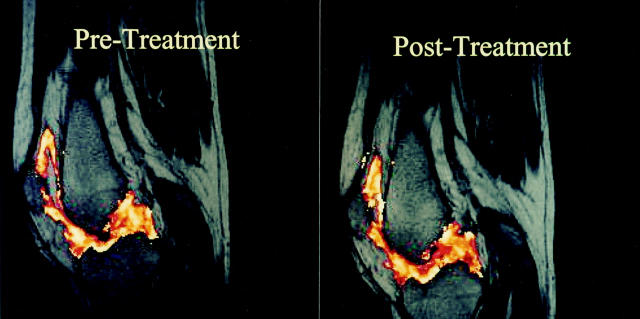
Figure 3 .
Quantitative maps of gadolinium-DTPA magnetic resonance images of the knee joint pre- and post-treatment with SB-210396. The images show the difference in maximal rate of signal intensity enhancement (sec-1 ) before and after treatment of one patient from the 40 mg treatment group. The scale ranges from minimum = 0.016 (red) to maximum = 0.88 (yellow). The pre-treatment image is predominantly yellow indicating a high maximal rate of enhancement while in contrast, the post-treatment image shows more red reflecting a reduced rate of enhancement .
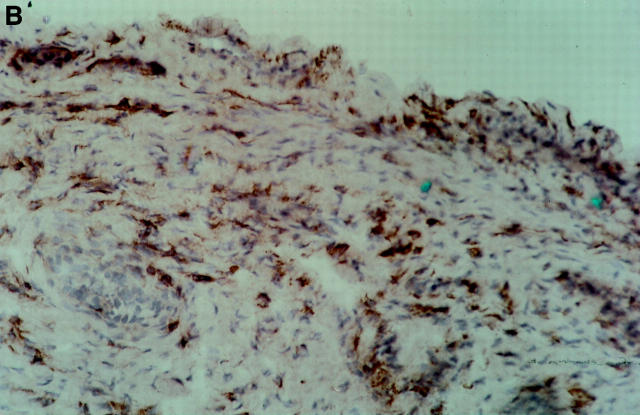
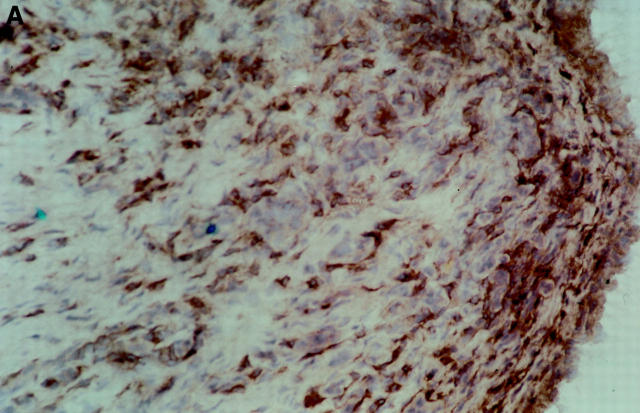
Figure 4 .
Photomicrographs of the synovial membrane before and after treatment. Immunoperoxidase staining with an anti-CD4 monoclonal antibody (OKT4), of a patient who received 40 mg active drug. (A) Synovial tissue before treatment shows staining of numerous CD4 + cells. (B) Synovial tissue after treatment from the same patient showing marked reduction of CD4+ cells.
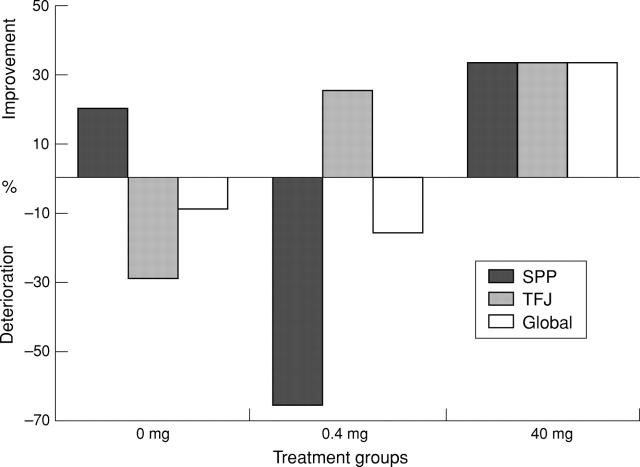
Figure 5 .
Percentage change in the median CD4 scores. This graph shows change from baseline in immunohistological CD4 scores at the regions of interest and globally, for the three treatment groups. Improvement (>30%) was seen at all sites in the 40 mg group. Improvement was also seen at the tibiofemoral joint region in the 0.4 mg group and at the suprapatellar region in the placebo group.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkengren A. G., Geborek P., Rydholm U., Holtås S., Petterson H. MR imaging of the knee in acute rheumatoid arthritis: synovial uptake of gadolinium-DOTA. AJR Am J Roentgenol. 1990 Aug;155(2):329–332. doi: 10.2214/ajr.155.2.2115261. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Chikanza I. C., Kingsley G. H., Corrigall V., Panayi G. S. Treatment of rheumatoid arthritis with single dose or weekly pulses of chimaeric anti-CD4 monoclonal antibody. Scand J Immunol. 1992 Aug;36(2):291–298. doi: 10.1111/j.1365-3083.1992.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Choy E. H., Pitzalis C., Cauli A., Bijl J. A., Schantz A., Woody J., Kingsley G. H., Panayi G. S. Percentage of anti-CD4 monoclonal antibody-coated lymphocytes in the rheumatoid joint is associated with clinical improvement. Implications for the development of immunotherapeutic dosing regimens. Arthritis Rheum. 1996 Jan;39(1):52–56. doi: 10.1002/art.1780390107. [DOI] [PubMed] [Google Scholar]
- Epstein W. V. Expectation bias in rheumatoid arthritis clinical trials. The anti-CD4 monoclonal antibody experience. Arthritis Rheum. 1996 Nov;39(11):1773–1780. doi: 10.1002/art.1780391102. [DOI] [PubMed] [Google Scholar]
- Foley-Nolan D., Stack J. P., Ryan M., Redmond U., Barry C., Ennis J., Coughlan R. J. Magnetic resonance imaging in the assessment of rheumatoid arthritis--a comparison with plain film radiographs. Br J Rheumatol. 1991 Apr;30(2):101–106. doi: 10.1093/rheumatology/30.2.101. [DOI] [PubMed] [Google Scholar]
- Gaffney K., Cookson J., Blake D., Coumbe A., Blades S. Quantification of rheumatoid synovitis by magnetic resonance imaging. Arthritis Rheum. 1995 Nov;38(11):1610–1617. doi: 10.1002/art.1780381113. [DOI] [PubMed] [Google Scholar]
- Gilkeson G., Polisson R., Sinclair H., Vogler J., Rice J., Caldwell D., Spritzer C., Martinez S. Early detection of carpal erosions in patients with rheumatoid arthritis: a pilot study of magnetic resonance imaging. J Rheumatol. 1988 Sep;15(9):1361–1366. [PubMed] [Google Scholar]
- Goldberg D., Morel P., Chatenoud L., Boitard C., Menkes C. J., Bertoye P. H., Revillard J. P., Bach J. F. Immunological effects of high dose administration of anti-CD4 antibody in rheumatoid arthritis patients. J Autoimmun. 1991 Aug;4(4):617–630. doi: 10.1016/0896-8411(91)90181-b. [DOI] [PubMed] [Google Scholar]
- Herzog C., Walker C., Müller W., Rieber P., Reiter C., Riethmüller G., Wassmer P., Stockinger H., Madic O., Pichler W. J. Anti-CD4 antibody treatment of patients with rheumatoid arthritis: I. Effect on clinical course and circulating T cells. J Autoimmun. 1989 Oct;2(5):627–642. doi: 10.1016/s0896-8411(89)80002-2. [DOI] [PubMed] [Google Scholar]
- Horneff G., Burmester G. R., Emmrich F., Kalden J. R. Treatment of rheumatoid arthritis with an anti-CD4 monoclonal antibody. Arthritis Rheum. 1991 Feb;34(2):129–140. doi: 10.1002/art.1780340202. [DOI] [PubMed] [Google Scholar]
- Ike R. W. Diagnostic arthroscopy. Baillieres Clin Rheumatol. 1996 Aug;10(3):495–517. doi: 10.1016/s0950-3579(96)80046-x. [DOI] [PubMed] [Google Scholar]
- König H., Sieper J., Wolf K. J. Rheumatoid arthritis: evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology. 1990 Aug;176(2):473–477. doi: 10.1148/radiology.176.2.2367663. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Bucy R. P., Tilden A., Pratt P. W., LoBuglio A. F., Khazaeli M., Everson M. P., Daddona P., Ghrayeb J., Kilgarriff C. Use of a chimeric monoclonal anti-CD4 antibody in patients with refractory rheumatoid arthritis. Arthritis Rheum. 1993 Mar;36(3):307–318. doi: 10.1002/art.1780360304. [DOI] [PubMed] [Google Scholar]
- Moreland L. W., Pratt P. W., Mayes M. D., Postlethwaite A., Weisman M. H., Schnitzer T., Lightfoot R., Calabrese L., Zelinger D. J., Woody J. N. Double-blind, placebo-controlled multicenter trial using chimeric monoclonal anti-CD4 antibody, cM-T412, in rheumatoid arthritis patients receiving concomitant methotrexate. Arthritis Rheum. 1995 Nov;38(11):1581–1588. doi: 10.1002/art.1780381109. [DOI] [PubMed] [Google Scholar]
- Ostergaard M., Stoltenberg M., Henriksen O., Lorenzen I. Quantitative assessment of synovial inflammation by dynamic gadolinium-enhanced magnetic resonance imaging. A study of the effect of intra-articular methylprednisolone on the rate of early synovial enhancement. Br J Rheumatol. 1996 Jan;35(1):50–59. doi: 10.1093/rheumatology/35.1.50. [DOI] [PubMed] [Google Scholar]
- Panayi G. S., Lanchbury J. S., Kingsley G. H. The importance of the T cell in initiating and maintaining the chronic synovitis of rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):729–735. doi: 10.1002/art.1780350702. [DOI] [PubMed] [Google Scholar]
- Paulus H. E., Machleder H. I., Levine S., Yu D. T., MacDonald N. S. Lymphocyte involvement in rheumatoid arthritis. Studies during thoracic duct drainage. Arthritis Rheum. 1977 Jul-Aug;20(6):1249–1262. doi: 10.1002/art.1780200614. [DOI] [PubMed] [Google Scholar]
- Reece R., Emery P. Needle arthroscopy. Br J Rheumatol. 1995 Dec;34(12):1102–1104. doi: 10.1093/rheumatology/34.12.1102. [DOI] [PubMed] [Google Scholar]
- Reiter C., Kakavand B., Rieber E. P., Schattenkirchner M., Riethmüller G., Krüger K. Treatment of rheumatoid arthritis with monoclonal CD4 antibody M-T151. Clinical results and immunopharmacologic effects in an open study, including repeated administration. Arthritis Rheum. 1991 May;34(5):525–536. doi: 10.1002/art.1780340504. [DOI] [PubMed] [Google Scholar]
- Robb R. A., Hanson D. P., Karwoski R. A., Larson A. G., Workman E. L., Stacy M. C. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and analysis. Comput Med Imaging Graph. 1989 Nov-Dec;13(6):433–454. doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Tak P. P., Taylor P. C., Breedveld F. C., Smeets T. J., Daha M. R., Kluin P. M., Meinders A. E., Maini R. N. Decrease in cellularity and expression of adhesion molecules by anti-tumor necrosis factor alpha monoclonal antibody treatment in patients with rheumatoid arthritis. Arthritis Rheum. 1996 Jul;39(7):1077–1081. doi: 10.1002/art.1780390702. [DOI] [PubMed] [Google Scholar]
- Tak P. P., van der Lubbe P. A., Cauli A., Daha M. R., Smeets T. J., Kluin P. M., Meinders A. E., Yanni G., Panayi G. S., Breedveld F. C. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum. 1995 Oct;38(10):1457–1465. doi: 10.1002/art.1780381012. [DOI] [PubMed] [Google Scholar]
- Tamai K., Yamato M., Yamaguchi T., Ohno W. Dynamic magnetic resonance imaging for the evaluation of synovitis in patients with rheumatoid arthritis. Arthritis Rheum. 1994 Aug;37(8):1151–1157. doi: 10.1002/art.1780370807. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Belli J. A., Anderson R. J., Buckley J. A., Goetzl E. J., David J. R., Austen K. F. Clinical and immunologic effects of fractionated total lymphoid irradiation in refractory rheumatoid arthritis. N Engl J Med. 1981 Oct 22;305(17):976–982. doi: 10.1056/NEJM198110223051703. [DOI] [PubMed] [Google Scholar]
- Tugwell P., Pincus T., Yocum D., Stein M., Gluck O., Kraag G., McKendry R., Tesser J., Baker P., Wells G. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. The Methotrexate-Cyclosporine Combination Study Group. N Engl J Med. 1995 Jul 20;333(3):137–141. doi: 10.1056/NEJM199507203330301. [DOI] [PubMed] [Google Scholar]
- Vincenti M. P., Coon C. I., White L. A., Barchowsky A., Brinckerhoff C. E. src-related tyrosine kinases regulate transcriptional activation of the interstitial collagenase gene, MMP-1, in interleukin-1-stimulated synovial fibroblasts. Arthritis Rheum. 1996 Apr;39(4):574–582. doi: 10.1002/art.1780390406. [DOI] [PubMed] [Google Scholar]
- Wendling D., Racadot E., Morel-Fourrier B., Wijdenes J. Treatment of rheumatoid arthritis with anti CD4 monoclonal antibody. Open study of 25 patients with the B-F5 clone. Clin Rheumatol. 1992 Dec;11(4):542–547. doi: 10.1007/BF02283116. [DOI] [PubMed] [Google Scholar]
- Yanni G., Nabil M., Farahat M. R., Poston R. N., Panayi G. S. Intramuscular gold decreases cytokine expression and macrophage numbers in the rheumatoid synovial membrane. Ann Rheum Dis. 1994 May;53(5):315–322. doi: 10.1136/ard.53.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lubbe P. A., Dijkmans B. A., Markusse H. M., Nässander U., Breedveld F. C. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum. 1995 Aug;38(8):1097–1106. doi: 10.1002/art.1780380812. [DOI] [PubMed] [Google Scholar]
- van der Lubbe P. A., Reiter C., Breedveld F. C., Krüger K., Schattenkirchner M., Sanders M. E., Riethmüller G. Chimeric CD4 monoclonal antibody cM-T412 as a therapeutic approach to rheumatoid arthritis. Arthritis Rheum. 1993 Oct;36(10):1375–1379. doi: 10.1002/art.1780361008. [DOI] [PubMed] [Google Scholar]