Abstract
OBJECTIVE—This study was designed to investigate whether 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3), produced by activated synovial fluid macrophages, promotes its own catabolism by upregulating vitamin D-24-hydroxylase (24-OHase) in synovial fibroblasts through a vitamin D receptor (VDR) mediated mechanism. METHODS—Synovial macrophages and fibroblasts were derived from patients with rheumatoid arthritis. Expression of VDR and 24-OHase mRNAs was determined using in situ hybridisation. Vitamin D hydroxylase activity was determined by incubating cells with [3H]-25-(OH)D3, or [3H]-1,25-(OH)2D3, and metabolite synthesis quantified using high performance liquid chromatography. RESULTS—1,25-(OH)2D3 increased expression of mRNA for both VDR and 24-OHase in fibroblasts by approximately threefold over 24 hours. 1,25-(OH)2D3 increased fibroblast 24-OHase activity, yielding 24-hydroxylated, and more polar, metabolites. In co-culture, fibroblasts were able to catabolise macrophage derived 1,25-(OH)2D3. CONCLUSIONS—1,25-(OH)2D3 is produced by macrophages in vitro at biologically relevant concentrations and can increase its own catabolism by synovial fibroblasts; this effect is probably mediated via upregulation of both synovial fibroblast VDR and 24-OHase.
Full Text
The Full Text of this article is available as a PDF (2.7 MB).
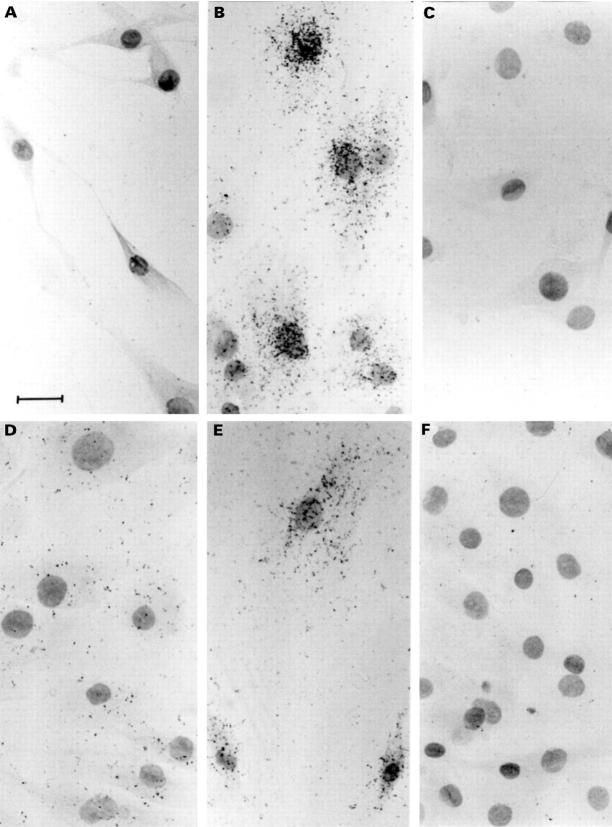
Figure 1 .
Photomicrographs showing hybridisation of [35S] labelled riboprobe for 24-OHase and VDR mRNAs in F733 synovial fibroblasts. F733 cells were pre-treated with 5 × 10-8 M 1,25-(OH)2D3, or vehicle (0.125% v/v ethanol), fixed, then hybridised overnight with [35S] labelled riboprobe for 24-OHase or VDR mRNA. (A) Twenty four hour vehicle treated cells, 24-OHase probe; (B) 24 hour 1,25-(OH) 2D3 treated cells, 24-OHase probe; (C) RNase A treated control slide, 24-OHase probe; (D) 24 hour vehicle treated cells, VDR probe; (E) 24 hour 1,25-(OH)2D3 treated cells, VDR probe; (F) RNase A treated control slide, VDR probe. Bar = 50 µm, cells counterstained with eosin and Harris haematoxylin (Sigma).
Figure 2 .
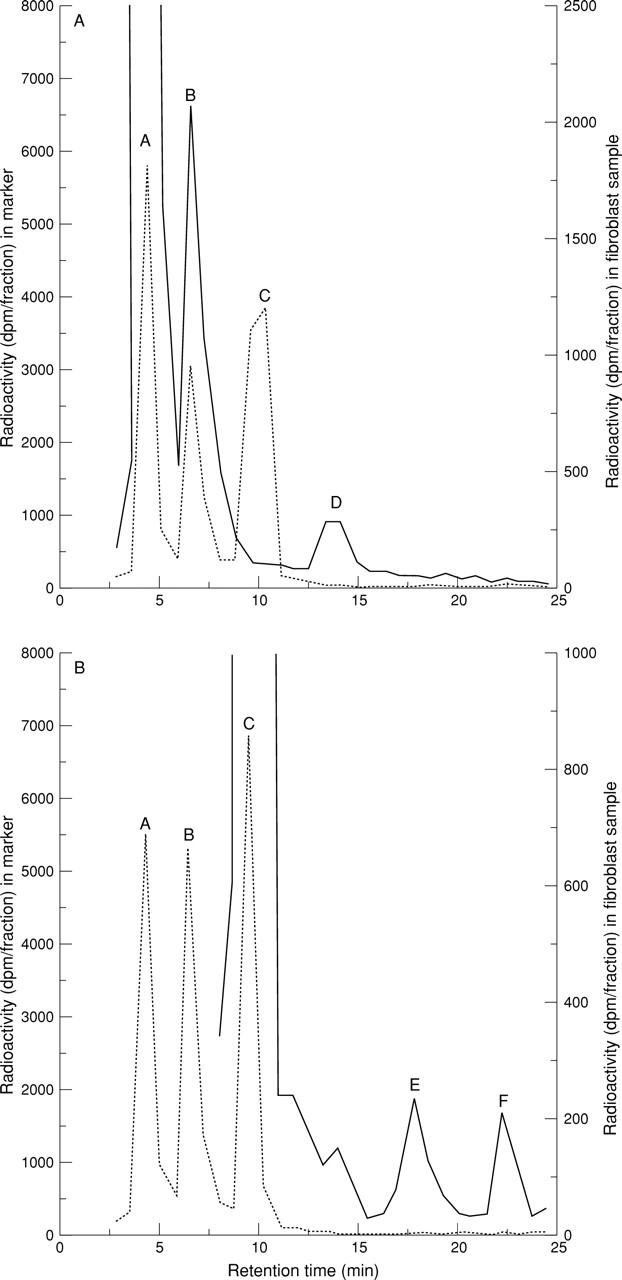
HPLC profile of synovial fluid fibroblast (A) [3H]-25-(OH)D3 and (B) [3H]-1,25-(OH)2D3 metabolism. HPLC conditions are as described in Methods. The dotted traces show the positions of known tritiated forms of 25-(OH)D3 (peak A), 24,25-(OH) 2D3 (peak B), and 1,25-(OH) 2D3 (peak C). As no radioactive tracer was available, the expected position of a tritiated 1,24,25-(OH) 3D3 peak was calculated using an ultraviolet trace of unlabelled markers in conjunction with the radioactive trace above. The solid trace in figure 2 (A) shows the metabolism of substrate [3H]-25-(OH)D3 to its 24-hydroxylated form, [3H]-24,25-(OH)2D3, by synovial fluid fibroblasts pre-treated for 24 hours with 10-8 M 1,25-(OH) 2D3. The position of an unidentified product, which was more polar than [3H]-24,25-(OH) 2D3, is indicated by peak D. The solid trace in figure 2 (B) shows the metabolism of substrate [3H]-1,25-(OH)2D3 by synovial fluid fibroblasts pre-treated for 24 hours with 10-11 M 1,25-(OH)2D3. A metabolite with an elution time representing that of [3H]-1,24,25-(OH)3D3 is shown by peak E and the position of an unidentified product, which was more polar than [3H]-1,24,25-(OH)3D3, is indicated by peak F; dpm = disintegrations per minute.
Figure 3 .
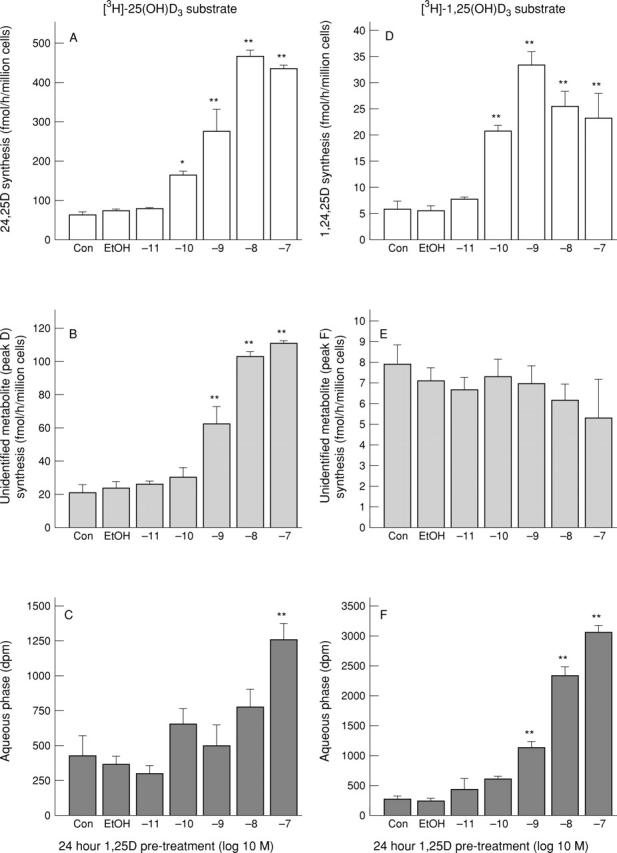
Effects of 1,25-(OH)2D3 pre-treatment on the metabolism of [3H]-25-(OH)D3 and [3H]-1,25-(OH)2D3 by F733 synovial fibroblasts. Panels (A-C) are cells incubated with [3H]-25-(OH)D3 substrate, panels (D-F) are cells incubated with [3H]-1,25-(OH)2D3 substrate. (A) Synthesis of [3H]-24,25-(OH)2D3; (B) synthesis of an unidentified product (peak D in fig 2 (A)); (C) the radioactivity present in the aqueous phase; (D) synthesis of [3H]-1,24,25-(OH)3D3; (E) synthesis of an unidentified product (peak F in figure 2 (B)) and (F) radioactivity present in the aqueous phase. n=3 for each point. Results are shown as mean (SEM). *p<0.05, **p<0.01 compared with vehicle (0.25% ethanol) using ANOVA followed by Dunnett's multiple comparison test.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Cantorna M. T., Hayes C. E., DeLuca H. F. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998 Jan;128(1):68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]
- Chen K. S., DeLuca H. F. Cloning of the human 1 alpha,25-dihydroxyvitamin D-3 24-hydroxylase gene promoter and identification of two vitamin D-responsive elements. Biochim Biophys Acta. 1995 Jul 25;1263(1):1–9. doi: 10.1016/0167-4781(95)00060-t. [DOI] [PubMed] [Google Scholar]
- Hayes M. E., Bayley D., Still P., Palit J., Denton J., Freemont A. J., Cooper R. G., Mawer E. B. Differential metabolism of 25-hydroxyvitamin D3 by cultured synovial fluid macrophages and fibroblast-like cells from patients with arthritis. Ann Rheum Dis. 1992 Feb;51(2):220–226. doi: 10.1136/ard.51.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. E., Denton J., Freemont A. J., Mawer E. B. Synthesis of the active metabolite of vitamin D, 1,25(OH)2D3, by synovial fluid macrophages in arthritic diseases. Ann Rheum Dis. 1989 Sep;48(9):723–729. doi: 10.1136/ard.48.9.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes M. E., Rai A., Cooper R. G., Bayley D., Freemont A. J., Mawer E. B. Inhibition by prostaglandin E1 and E2 of 1,25-dihydroxyvitamin D3 synthesis by synovial fluid macrophages from arthritic joints. Ann Rheum Dis. 1992 May;51(5):632–637. doi: 10.1136/ard.51.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Kleiner-Bossaller A., Schnoes H. K., Kasten P. M., Boyle I. T., DeLuca H. F. 1,24,25-Trihydroxyvitamin D3. A metabolite of vitamin D3 effective on intestine. J Biol Chem. 1973 Oct 10;248(19):6691–6696. [PubMed] [Google Scholar]
- Huckins D., Felson D. T., Holick M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: a pilot study. Arthritis Rheum. 1990 Nov;33(11):1723–1727. doi: 10.1002/art.1780331117. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Archer D. C. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991 Mar;87(3):1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C., Laureys J., Sobis H., Vandeputte M., Waer M., Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes. 1992 Nov;41(11):1491–1495. doi: 10.2337/diab.41.11.1491. [DOI] [PubMed] [Google Scholar]
- Mathieu C., Waer M., Casteels K., Laureys J., Bouillon R. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology. 1995 Mar;136(3):866–872. doi: 10.1210/endo.136.3.7867594. [DOI] [PubMed] [Google Scholar]
- Mawer E. B., Berry J. L., Cundall J. P., Still P. E., White A. A sensitive radioimmunoassay using a monoclonal antibody that is equipotent for ercalcitriol and calcitriol (1,25-dihydroxy vitamin D2 and D3). Clin Chim Acta. 1990 Oct 15;190(3):199–209. doi: 10.1016/0009-8981(90)90174-q. [DOI] [PubMed] [Google Scholar]
- Mawer E. B., Hayes M. E., Still P. E., Davies M., Lumb G. A., Palit J., Holt P. J. Evidence for nonrenal synthesis of 1,25-dihydroxyvitamin D in patients with inflammatory arthritis. J Bone Miner Res. 1991 Jul;6(7):733–739. doi: 10.1002/jbmr.5650060711. [DOI] [PubMed] [Google Scholar]
- Mee A. P., Denton J., Hoyland J. A., Davies M., Mawer E. B. Quantification of vitamin D receptor mRNA in tissue sections demonstrates the relative limitations of in situ-reverse transcriptase-polymerase chain reaction. J Pathol. 1997 May;182(1):22–28. doi: 10.1002/(SICI)1096-9896(199705)182:1<22::AID-PATH809>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Mee A. P., Webber D. M., May C., Bennett D., Sharpe P. T., Anderson D. C. Detection of canine distemper virus in bone cells in the metaphyses of distemper-infected dogs. J Bone Miner Res. 1992 Jul;7(7):829–834. doi: 10.1002/jbmr.5650070712. [DOI] [PubMed] [Google Scholar]
- Tsuji M., Fujii K., Nakano T., Nishii Y. 1 alpha-hydroxyvitamin D3 inhibits type II collagen-induced arthritis in rats. FEBS Lett. 1994 Jan 17;337(3):248–250. doi: 10.1016/0014-5793(94)80201-7. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Tetlow L. C. Observations on the microenvironmental nature of cartilage degradation in rheumatoid arthritis. Ann Rheum Dis. 1997 Mar;56(3):151–161. doi: 10.1136/ard.56.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. Y., Freemont A. J., Mawer E. B., Hayes M. E. Regulation of 1 alpha, 25-dihydroxyvitamin D3 synthesis in macrophages from arthritic joints by phorbol ester, dibutyryl-cAMP and calcium ionophore (A23187). FEBS Lett. 1992 Oct 12;311(1):71–74. doi: 10.1016/0014-5793(92)81370-2. [DOI] [PubMed] [Google Scholar]