Abstract
OBJECTIVE—Urokinase type plasminogen activator (uPA) catalyses the formation of the proteolytic enzyme plasmin, which is involved in matrix degradation in the processes of tissue remodelling. Because of a surface bound uPA receptor (uPAR), expressed by some cell types (for example, macrophages, malignant cells and inflammatory activated synoviocytes), the action of uPA can be localised and intensified. uPAR seems to have a role in the mechanisms leading to invasive growth of malignant tissue and the rheumatoid pannus. uPAR may become cleaved at its cell surface anchor, thus forming a free soluble receptor (suPAR). suPAR is detectable in low but constant values in plasma of healthy people, while increased concentrations are found in patients with disseminated malignant disease, so that suPAR may be an indicator of invasive growth and tissue remodelling. suPAR concentrations in plasma have not previously been measured in rheumatic patients. A controlled cross sectional measurement was performed of suPAR in plasma of patients with various inflammatory rheumatic disorders with special reference to rheumatoid arthritis (RA). METHODS—suPAR in plasma was measured by ELISA technique in patients with RA (n=51), reactive arthritis (ReA) (n=23), primary Sjögren's syndrome (PSS) (n=42) and sex and age matched healthy controls (n=53). RESULTS—In the control group suPAR (median) was 0.91 (range 0.56-1.94) µg/l. Median suPAR value in RA was 1.47 (range 0.65-6.62) µg/l; in ReA 0.68 µg/l (range 0.52-1.48) and in PSS 1.12 µg/l (range 0.76-1.92); p versus controls <0.001 in all patient groups. suPAR values in RA were also significantly increased compared with ReA (p<0.001) and PSS (p=0.004) groups. suPAR in RA was positively correlated to C reactive protein (CRP) (p<0.01) and erythrocyte sedimentation rate (p<0.05) and number of swollen joints (p<0.05). The ReA group had the highest CRP values of all groups, but at the same time the lowest suPAR concentrations in plasma. CONCLUSIONS—Increased suPAR concentrations were found in plasma in RA, and to a smaller extent also in PSS, but not in ReA. In RA suPAR is related to disease activity. suPAR seems though not merely to be an acute phase reactant like CRP. Increased suPAR values might reflect erosive activity in RA.
Full Text
The Full Text of this article is available as a PDF (74.7 KB).
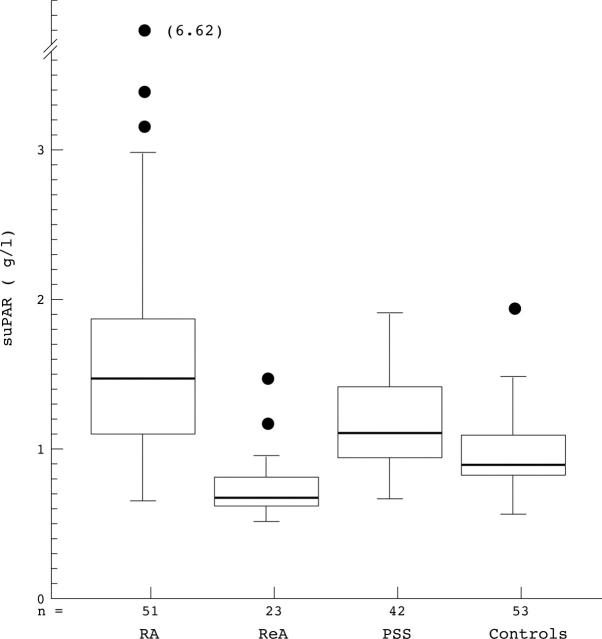
Figure 1 .
suPAR concentrations in different patient groups shown in box plots. Medians (heavy lines in boxes) and interquartile range are indicated as boxes. Whiskers indicates total range excluding outliers, which are shown separately. RA: rheumatoid arthritis. ReA: reactive arthritis. PSS: primary Sjögren's syndrome.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Belcher C., Fawthrop F., Bunning R., Doherty M. Plasminogen activators and their inhibitors in synovial fluids from normal, osteoarthritis, and rheumatoid arthritis knees. Ann Rheum Dis. 1996 Apr;55(4):230–236. doi: 10.1136/ard.55.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommer E. J., Dooijewaard G., Dijkmans B. A., Breedveld F. C. Depression of tissue-type plasminogen activator and enhancement of urokinase-type plasminogen activator as an expression of local inflammation. Thromb Haemost. 1992 Aug 3;68(2):180–184. [PubMed] [Google Scholar]
- Brommer E. J., Dooijewaard G., Dijkmans B. A., Breedveld F. C. Plasminogen activators in synovial fluid and plasma from patients with arthritis. Ann Rheum Dis. 1992 Aug;51(8):965–968. doi: 10.1136/ard.51.8.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N., Péclat V., So A., Sappino A. P. Plasminogen activation in synovial tissues: differences between normal, osteoarthritis, and rheumatoid arthritis joints. Ann Rheum Dis. 1997 Sep;56(9):550–557. doi: 10.1136/ard.56.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N., Péclat V., Van Ness K., Kolodziesczyk E., Degen J., Bugge T., So A. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998 Jul 1;102(1):41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busso N., So A. Urokinase in rheumatoid arthritis: causal or coincidental? Ann Rheum Dis. 1997 Dec;56(12):705–706. doi: 10.1136/ard.56.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K., Piccoli D. S., Roberts M. J., Muirden K. D., Hamilton J. A. Effects of tumor necrosis factor alpha and beta on resorption of human articular cartilage and production of plasminogen activator by human articular chondrocytes. Arthritis Rheum. 1990 Apr;33(4):542–552. doi: 10.1002/art.1780330412. [DOI] [PubMed] [Google Scholar]
- Cerinic M. M., Generini S., Partsch G., Pignone A., Dini G., Konttinen Y. T., Del Rosso M. Synoviocytes from osteoarthritis and rheumatoid arthritis produce plasminogen activators and plasminogen activator inhibitor-1 and display u-PA receptors on their surface. Life Sci. 1998;63(6):441–453. doi: 10.1016/s0024-3205(98)00293-8. [DOI] [PubMed] [Google Scholar]
- Del Rosso M., Fibbi G., Pucci M., Cerinic M. M. Antisense oligonucleotides against the urokinase receptor: a therapeutic strategy for the control of cell invasion in rheumatoid arthritis and cancer. Clin Exp Rheumatol. 1998 Jul-Aug;16(4):389–393. [PubMed] [Google Scholar]
- Fibbi G., Pucci M., Serni U., Cerinic M. M., Del Rosso M. Antisense targeting of the urokinase receptor blocks urokinase-dependent proliferation, chemoinvasion, and chemotaxis of human synovial cells and chondrocytes in vitro. Proc Assoc Am Physicians. 1998 Jul-Aug;110(4):340–350. [PubMed] [Google Scholar]
- Kikuchi H., Tanaka S., Matsuo O. Plasminogen activator in synovial fluid from patients with rheumatoid arthritis. J Rheumatol. 1987 Jun;14(3):439–445. [PubMed] [Google Scholar]
- Kirchheimer J. C., Remold H. G., Wanivenhaus A., Binder B. R. Increased proteolytic activity on the surface of monocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1430–1433. doi: 10.1002/art.1780341114. [DOI] [PubMed] [Google Scholar]
- Kummer J. A., Abbink J. J., de Boer J. P., Roem D., Nieuwenhuys E. J., Kamp A. M., Swaak T. J., Hack C. E. Analysis of intraarticular fibrinolytic pathways in patients with inflammatory and noninflammatory joint diseases. Arthritis Rheum. 1992 Aug;35(8):884–893. doi: 10.1002/art.1780350806. [DOI] [PubMed] [Google Scholar]
- Mochan E., Uhl J. Elevations in synovial fluid plasminogen activator in patients with rheumatoid arthritis. J Rheumatol. 1984 Apr;11(2):123–128. [PubMed] [Google Scholar]
- Romer J., Bugge T. H., Pyke C., Lund L. R., Flick M. J., Degen J. L., Dano K. Impaired wound healing in mice with a disrupted plasminogen gene. Nat Med. 1996 Mar;2(3):287–292. doi: 10.1038/nm0396-287. [DOI] [PubMed] [Google Scholar]
- Ronday H. K., Smits H. H., Quax P. H., van der Pluijm G., Löwik C. W., Breedveld F. C., Verheijen J. H. Bone matrix degradation by the plasminogen activation system. Possible mechanism of bone destruction in arthritis. Br J Rheumatol. 1997 Jan;36(1):9–15. doi: 10.1093/rheumatology/36.1.9. [DOI] [PubMed] [Google Scholar]
- Ronday H. K., Smits H. H., Van Muijen G. N., Pruszczynski M. S., Dolhain R. J., Van Langelaan E. J., Breedveld F. C., Verheijen J. H. Difference in expression of the plasminogen activation system in synovial tissue of patients with rheumatoid arthritis and osteoarthritis. Br J Rheumatol. 1996 May;35(5):416–423. doi: 10.1093/rheumatology/35.5.416. [DOI] [PubMed] [Google Scholar]
- Rønne E., Pappot H., Grøndahl-Hansen J., Høyer-Hansen G., Plesner T., Hansen N. E., Danø K. The receptor for urokinase plasminogen activator is present in plasma from healthy donors and elevated in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1995 Mar;89(3):576–581. doi: 10.1111/j.1365-2141.1995.tb08366.x. [DOI] [PubMed] [Google Scholar]
- Saxne T., Lecander I., Geborek P. Plasminogen activators and plasminogen activator inhibitors in synovial fluid. Difference between inflammatory joint disorders and osteoarthritis. J Rheumatol. 1993 Jan;20(1):91–96. [PubMed] [Google Scholar]
- Stephens R. W., Pedersen A. N., Nielsen H. J., Hamers M. J., Høyer-Hansen G., Rønne E., Dybkjaer E., Danø K., Brünner N. ELISA determination of soluble urokinase receptor in blood from healthy donors and cancer patients. Clin Chem. 1997 Oct;43(10):1868–1876. [PubMed] [Google Scholar]
- Szekanecz Z., Haines G. K., Koch A. E. Differential expression of the urokinase receptor (CD87) in arthritic and normal synovial tissues. J Clin Pathol. 1997 Apr;50(4):314–319. doi: 10.1136/jcp.50.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Sappino A. P., Belin D. The plasminogen activator/plasmin system. J Clin Invest. 1991 Oct;88(4):1067–1072. doi: 10.1172/JCI115405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C., Bombardieri S., Moutsopoulos H. M., Balestrieri G., Bencivelli W., Bernstein R. M., Bjerrum K. B., Braga S., Coll J., de Vita S. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993 Mar;36(3):340–347. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- Zacharski L. R., Brown F. E., Memoli V. A., Kisiel W., Kudryk B. J., Rousseau S. M., Hunt J. A., Dunwiddie C., Nutt E. M. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992 May;63(2):155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]