Full Text
The Full Text of this article is available as a PDF (101.0 KB).
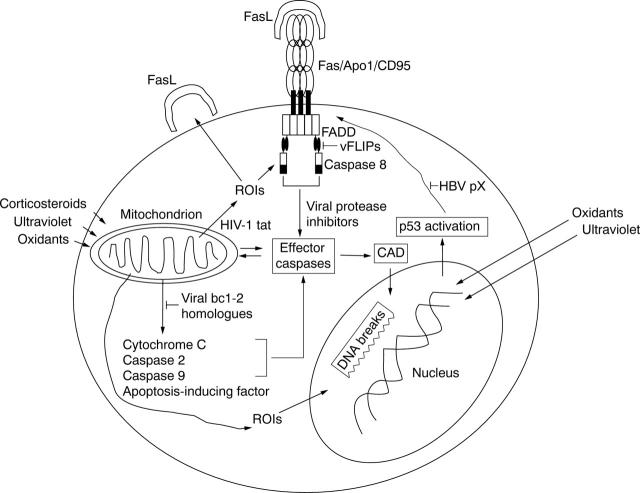
Figure 1 .
Regulation of apoptosis pathways by viral proteins. Oxidants, ultra violet light, and corticosteroids trigger apoptosis by mitochondrial damage, which, in turn, leads to the release of caspase activating factors.112 This process is inhibited by bcl-2 and its viral homologues. Release of reactive oxygen intermediates causes increased production of FasL and DNA fragmentation. Fas ligand (FasL) crosslinks the Fas receptor (Fas/Apo1/CD95), which recruits an adapter protein with a Fas associated death domain (FADD). Viral FLIPs (vFLIPs) possess a death effector domain similar to those of FADD and caspase 8 and, thus, interrupt Fas signalling. While not shown, vFLIPs may also block TNF receptor mediated signalling through FADD shared by both the Fas and TNF pathways.113 Upon recruitment of caspase 8, its oligomerisation causes self cleavage and activation of downstream effector caspases.113 A caspase activated DN-ase cleaves chromosomal DNA. Oxidants and ultra violet light as well as ROI released from damaged mitochondria cause DNA fragmentation, which in turn activates p53. p53 induces oxidative stress and augments surface expression of the Fas receptor. HIV-1 tat increases mitochondrial ROI production thus increasing apoptosis. By contrast HBV pX protein interferes with the proapoptotic effects of p53.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akriviadis E. A., Xanthakis I., Navrozidou C., Papadopoulos A. Prevalence of cryoglobulinemia in chronic hepatitis C virus infection and response to treatment with interferon-alpha. J Clin Gastroenterol. 1997 Dec;25(4):612–618. doi: 10.1097/00004836-199712000-00013. [DOI] [PubMed] [Google Scholar]
- Albani S., Carson D. A. A multistep molecular mimicry hypothesis for the pathogenesis of rheumatoid arthritis. Immunol Today. 1996 Oct;17(10):466–470. doi: 10.1016/0167-5699(96)20029-g. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Reveille J. D. Genetics of systemic lupus erythematosus. Rheum Dis Clin North Am. 1992 Nov;18(4):865–892. [PubMed] [Google Scholar]
- Banki K., Hutter E., Colombo E., Gonchoroff N. J., Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J Biol Chem. 1996 Dec 20;271(51):32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- Banki K., Hutter E., Gonchoroff N. J., Perl A. Molecular ordering in HIV-induced apoptosis. Oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. J Biol Chem. 1998 May 8;273(19):11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- Banki K., Maceda J., Hurley E., Ablonczy E., Mattson D. H., Szegedy L., Hung C., Perl A. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz S. R., Rogel M. E., Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996 Apr;70(4):2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilke M. A., Traina-Dorge V., England J. D., Blanchard J. L. Polymyositis, arthritis, and uveitis in a macaque experimentally infected with human T lymphotropic virus type I. Arthritis Rheum. 1996 Apr;39(4):610–615. doi: 10.1002/art.1780390410. [DOI] [PubMed] [Google Scholar]
- Bengtsson A., Blomberg J., Nived O., Pipkorn R., Toth L., Sturfelt G. Selective antibody reactivity with peptides from human endogenous retroviruses and nonviral poly(amino acids) in patients with systemic lupus erythematosus. Arthritis Rheum. 1996 Oct;39(10):1654–1663. doi: 10.1002/art.1780391007. [DOI] [PubMed] [Google Scholar]
- Bertin J., Armstrong R. C., Ottilie S., Martin D. A., Wang Y., Banks S., Wang G. H., Senkevich T. G., Alnemri E. S., Moss B. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes S. M., Pandolfino Y. A., Mitchell T. J., Venables P. J., Shattles W. G., Clark D. A., Entwistle A., Maini R. N. The immune response to and expression of cross-reactive retroviral gag sequences in autoimmune disease. Br J Rheumatol. 1992 Nov;31(11):735–742. doi: 10.1093/rheumatology/31.11.735. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. The E3/19K protein of adenovirus type 2 binds to the domains of histocompatibility antigens required for CTL recognition. EMBO J. 1987 Jul;6(7):2019–2026. doi: 10.1002/j.1460-2075.1987.tb02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese L. H. The rheumatic manifestations of infection with the human immunodeficiency virus. Semin Arthritis Rheum. 1989 May;18(4):225–239. doi: 10.1016/0049-0172(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen L. A., Anhalt G., Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994 Apr 1;179(4):1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheevers W. P., Beyer J. C., Knowles D. P. Type 1 and type 2 cytokine gene expression by viral gp135 surface protein-activated T lymphocytes in caprine arthritis-encephalitis lentivirus infection. J Virol. 1997 Aug;71(8):6259–6263. doi: 10.1128/jvi.71.8.6259-6263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Clerici M., Shearer G. M. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994 Dec;15(12):575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Collette Y., Chang H. L., Cerdan C., Chambost H., Algarte M., Mawas C., Imbert J., Burny A., Olive D. Specific Th1 cytokine down-regulation associated with primary clinically derived human immunodeficiency virus type 1 Nef gene-induced expression. J Immunol. 1996 Jan 1;156(1):360–370. [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Dayal A. K., Kammer G. M. The T cell enigma in lupus. Arthritis Rheum. 1996 Jan;39(1):23–33. doi: 10.1002/art.1780390104. [DOI] [PubMed] [Google Scholar]
- Ehret A., Westendorp M. O., Herr I., Debatin K. M., Heeney J. L., Frank R., Krammer P. H. Resistance of chimpanzee T cells to human immunodeficiency virus type 1 Tat-enhanced oxidative stress and apoptosis. J Virol. 1996 Sep;70(9):6502–6507. doi: 10.1128/jvi.70.9.6502-6507.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Niebur J., Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994 Apr 1;152(7):3685–3692. [PubMed] [Google Scholar]
- Ferri C., Greco F., Longombardo G., Palla P., Moretti A., Marzo E., Mazzoni A., Pasero G., Bombardieri S., Highfield P. Association between hepatitis C virus and mixed cryoglobulinemia [see comment]. Clin Exp Rheumatol. 1991 Nov-Dec;9(6):621–624. [PubMed] [Google Scholar]
- Ferri C., La Civita L., Longombardo G., Zignego A. L., Pasero G. Mixed cryoglobulinaemia: a cross-road between autoimmune and lymphoproliferative disorders. Lupus. 1998;7(4):275–279. doi: 10.1191/096120398678920091. [DOI] [PubMed] [Google Scholar]
- Ferri C., Monti M., La Civita L., Longombardo G., Greco F., Pasero G., Gentilini P., Bombardieri S., Zignego A. L. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993 Dec 15;82(12):3701–3704. [PubMed] [Google Scholar]
- Flexman J. P., Smith D. W., Mackenzie J. S., Fraser J. R., Bass S. P., Hueston L., Lindsay M. D., Cunningham A. L. A comparison of the diseases caused by Ross River virus and Barmah Forest virus. Med J Aust. 1998 Aug 3;169(3):159–163. doi: 10.5694/j.1326-5377.1998.tb116019.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Becker G. J. Mononuclear cell types in chronic synovial effusions of Ross River virus disease. Aust N Z J Med. 1984 Aug;14(4):505–506. doi: 10.1111/j.1445-5994.1984.tb03629.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. R. Epidemic polyarthritis and Ross River virus disease. Clin Rheum Dis. 1986 Aug;12(2):369–388. [PubMed] [Google Scholar]
- Fraser J. R., Ratnamohan V. M., Dowling J. P., Becker G. J., Varigos G. A. The exanthem of Ross River virus infection: histology, location of virus antigen and nature of inflammatory infiltrate. J Clin Pathol. 1983 Nov;36(11):1256–1263. doi: 10.1136/jcp.36.11.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser K. J., Clarris B. J., Muirden K. D., Fraser J. R., Jack I. A persistent adenovirus type 1 infection in synovial tissue from an immunodeficient patient with chronic, rheumatoid-like polyarthritis. Arthritis Rheum. 1985 Apr;28(4):455–458. doi: 10.1002/art.1780280416. [DOI] [PubMed] [Google Scholar]
- Frenkel L. M., Nielsen K., Garakian A., Jin R., Wolinsky J. S., Cherry J. D. A search for persistent rubella virus infection in persons with chronic symptoms after rubella and rubella immunization and in patients with juvenile rheumatoid arthritis. Clin Infect Dis. 1996 Feb;22(2):287–294. doi: 10.1093/clinids/22.2.287. [DOI] [PubMed] [Google Scholar]
- Gao J. L., Murphy P. M. Human cytomegalovirus open reading frame US28 encodes a functional beta chemokine receptor. J Biol Chem. 1994 Nov 18;269(46):28539–28542. [PubMed] [Google Scholar]
- Gendelman R., Orzech Y., Mashiah P., Birenbaum M., Gazit A., Yaniv A. Productive replication of caprine arthritis-encephalitis virus is associated with induction of apoptosis. J Gen Virol. 1997 Apr;78(Pt 4):801–805. doi: 10.1099/0022-1317-78-4-801. [DOI] [PubMed] [Google Scholar]
- Gorevic P. D., Kassab H. J., Levo Y., Kohn R., Meltzer M., Prose P., Franklin E. C. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980 Aug;69(2):287–308. doi: 10.1016/0002-9343(80)90390-3. [DOI] [PubMed] [Google Scholar]
- Green D. R., Reed J. C. Mitochondria and apoptosis. Science. 1998 Aug 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Green J. E., Hinrichs S. H., Vogel J., Jay G. Exocrinopathy resembling Sjögren's syndrome in HTLV-1 tax transgenic mice. Nature. 1989 Sep 7;341(6237):72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- Guérin B., Arfi S., Numéric P., Jean-Baptiste G., Le Parc J. M., Smadja D., Grollier-Bois L. Polyarthritis in HTLV-1-infected patients. A review of 17 cases. Rev Rhum Engl Ed. 1995 Jan;62(1):21–28. [PubMed] [Google Scholar]
- Haraguchi S., Good R. A., Day N. K. Immunosuppressive retroviral peptides: cAMP and cytokine patterns. Immunol Today. 1995 Dec;16(12):595–603. doi: 10.1016/0167-5699(95)80083-2. [DOI] [PubMed] [Google Scholar]
- Henderson S., Huen D., Rowe M., Dawson C., Johnson G., Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A., Jugovic P., York I., Russ G., Bennink J., Yewdell J., Ploegh H., Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995 Jun 1;375(6530):411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- Hinshaw V. S., Olsen C. W., Dybdahl-Sissoko N., Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994 Jun;68(6):3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M. C. Systemic lupus erythematosus. Rheum Dis Clin North Am. 1990 Aug;16(3):617–639. [PubMed] [Google Scholar]
- Iwakura Y., Tosu M., Yoshida E., Takiguchi M., Sato K., Kitajima I., Nishioka K., Yamamoto K., Takeda T., Hatanaka M. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science. 1991 Aug 30;253(5023):1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- Jackson J. M., Callen J. P. Scarring alopecia and sclerodermatous changes of the scalp in a patient with hepatitis C infection. J Am Acad Dermatol. 1998 Nov;39(5 Pt 2):824–826. doi: 10.1016/s0190-9622(98)70357-3. [DOI] [PubMed] [Google Scholar]
- James J. A., Kaufman K. M., Farris A. D., Taylor-Albert E., Lehman T. J., Harley J. B. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997 Dec 15;100(12):3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi S., Saijyo S., Arai M., Karasawa S., Ueda S., Kannagi M., Iwakura Y., Fujii M., Yonehara S. Resistance to fas-mediated apoptosis of peripheral T cells in human T lymphocyte virus type I (HTLV-I) transgenic mice with autoimmune arthropathy. J Exp Med. 1997 Jul 7;186(1):57–64. doi: 10.1084/jem.186.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka H., Yamamoto K., Fujii H., Miura H., Miyasaka N., Nishioka K., Miyamoto T. Fine epitope mapping of the human SS-B/La protein. Identification of a distinct autoepitope homologous to a viral gag polyprotein. J Clin Invest. 1990 May;85(5):1566–1574. doi: 10.1172/JCI114606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Gourley M. F., Perl A. Endogenous retroviruses: potential etiologic agents in autoimmunity. FASEB J. 1992 May;6(8):2537–2544. doi: 10.1096/fasebj.6.8.1592206. [DOI] [PubMed] [Google Scholar]
- Le Goff P., Chicault P., Saraux A., Baron D., Valls-Bellec I., Leroy J. P. Lymphoma with regression after methotrexate withdrawal in a patient with rheumatoid arthritis. Role for the Epstein-Barr virus. Rev Rhum Engl Ed. 1998 Apr;65(4):283–286. [PubMed] [Google Scholar]
- Lechner F., Vogt H. R., Seow H. F., Bertoni G., Cheevers W. P., von Bodungen U., Zurbriggen A., Peterhans E. Expression of cytokine mRNA in lentivirus-induced arthritis. Am J Pathol. 1997 Oct;151(4):1053–1065. [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Fan W. S., Horsfall A. C., Anderson A. C., Rigby S., Larsson E., Venables P. J. The expression of human endogenous retrovirus-3 in fetal cardiac tissue and antibodies in congenital heart block. Clin Exp Immunol. 1996 Jun;104(3):388–393. [PubMed] [Google Scholar]
- Li Q., Turk S. M., Hutt-Fletcher L. M. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J Virol. 1995 Jul;69(7):3987–3994. doi: 10.1128/jvi.69.7.3987-3994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisitsyn N., Lisitsyn N., Wigler M. Cloning the differences between two complex genomes. Science. 1993 Feb 12;259(5097):946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- Lunardi C., Tiso M., Borgato L., Nanni L., Millo R., De Sandre G., Severi A. B., Puccetti A. Chronic parvovirus B19 infection induces the production of anti-virus antibodies with autoantigen binding properties. Eur J Immunol. 1998 Mar;28(3):936–948. doi: 10.1002/(SICI)1521-4141(199803)28:03<936::AID-IMMU936>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Masutani H., Hirota K., Sasada T., Ueda-Taniguchi Y., Taniguchi Y., Sono H., Yodoi J. Transactivation of an inducible anti-oxidative stress protein, human thioredoxin by HTLV-I Tax. Immunol Lett. 1996 Dec;54(2-3):67–71. doi: 10.1016/s0165-2478(96)02651-x. [DOI] [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Misaki Y., Yamamoto K., Yanagi K., Miura H., Ichijo H., Kato T., Mato T., Welling-Wester S., Nishioka K., Ito K. B cell epitope on the U1 snRNP-C autoantigen contains a sequence similar to that of the herpes simplex virus protein. Eur J Immunol. 1993 May;23(5):1064–1071. doi: 10.1002/eji.1830230513. [DOI] [PubMed] [Google Scholar]
- Morey A. L., Ferguson D. J., Fleming K. A. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: evidence of cell death by apoptosis. J Pathol. 1993 Feb;169(2):213–220. doi: 10.1002/path.1711690207. [DOI] [PubMed] [Google Scholar]
- Mossman K., Upton C., McFadden G. The myxoma virus-soluble interferon-gamma receptor homolog, M-T7, inhibits interferon-gamma in a species-specific manner. J Biol Chem. 1995 Feb 17;270(7):3031–3038. doi: 10.1074/jbc.270.7.3031. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Naides S. J. Rheumatic manifestations of parvovirus B19 infection. Rheum Dis Clin North Am. 1998 May;24(2):375–401. doi: 10.1016/s0889-857x(05)70014-4. [DOI] [PubMed] [Google Scholar]
- Narayan O., Cork L. C. Lentiviral diseases of sheep and goats: chronic pneumonia leukoencephalomyelitis and arthritis. Rev Infect Dis. 1985 Jan-Feb;7(1):89–98. doi: 10.1093/clinids/7.1.89. [DOI] [PubMed] [Google Scholar]
- Natkunam Y., Elenitoba-Johnson K. S., Kingma D. W., Kamel O. W. Epstein-Barr virus strain type and latent membrane protein 1 gene deletions in lymphomas in patients with rheumatic diseases. Arthritis Rheum. 1997 Jun;40(6):1152–1156. doi: 10.1002/art.1780400621. [DOI] [PubMed] [Google Scholar]
- Nauclér C. S., Larsson S., Möller E. A novel mechanism for virus-induced autoimmunity in humans. Immunol Rev. 1996 Aug;152:175–192. doi: 10.1111/j.1600-065x.1996.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Nishioka K. HTLV-I arthropathy and Sjögren syndrome. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13 (Suppl 1):S57–S62. doi: 10.1097/00042560-199600001-00011. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Panush R. S. Adenovirus arthritis. Arthritis Rheum. 1974 Sep-Oct;17(5):534–536. doi: 10.1002/art.1780170507. [DOI] [PubMed] [Google Scholar]
- Paxton W. A., Dragic T., Koup R. A., Moore J. P. The beta-chemokines, HIV type 1 second receptors, and exposed uninfected persons. AIDS Res Hum Retroviruses. 1996 Sep 1;12(13):1203–1207. doi: 10.1089/aid.1996.12.1203. [DOI] [PubMed] [Google Scholar]
- Perl A., Banki K. Human endogenous retroviral elements and autoimmunity: data and concepts. Trends Microbiol. 1993 Jul;1(4):153–156. doi: 10.1016/0966-842x(93)90131-a. [DOI] [PubMed] [Google Scholar]
- Perl A., Colombo E., Dai H., Agarwal R., Mark K. A., Banki K., Poiesz B. J., Phillips P. E., Hoch S. O., Reveille J. D. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes. Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995 Nov;38(11):1660–1671. doi: 10.1002/art.1780381119. [DOI] [PubMed] [Google Scholar]
- Perl A., Gorevic P. D., Ryan D. H., Condemi J. J., Ruszkowski R. J., Abraham G. N. Clonal B cell expansions in patients with essential mixed cryoglobulinaemia. Clin Exp Immunol. 1989 Apr;76(1):54–60. [PMC free article] [PubMed] [Google Scholar]
- Perl A., Isaacs C. M., Eddy R. L., Byers M. G., Sait S. N., Shows T. B. The human T-cell leukemia virus-related endogenous sequence (HRES1) is located on chromosome 1 at q42. Genomics. 1991 Dec;11(4):1172–1173. doi: 10.1016/0888-7543(91)90052-g. [DOI] [PubMed] [Google Scholar]
- Perl A., Rosenblatt J. D., Chen I. S., DiVincenzo J. P., Bever R., Poiesz B. J., Abraham G. N. Detection and cloning of new HTLV-related endogenous sequences in man. Nucleic Acids Res. 1989 Sep 12;17(17):6841–6854. doi: 10.1093/nar/17.17.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E. Viral arthritis. Curr Opin Rheumatol. 1997 Jul;9(4):337–344. doi: 10.1097/00002281-199707000-00011. [DOI] [PubMed] [Google Scholar]
- Planelles V., Jowett J. B., Li Q. X., Xie Y., Hahn B., Chen I. S. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J Virol. 1996 Apr;70(4):2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proffitt J. L., Sharma E., Blair G. E. Adenovirus 12-mediated down-regulation of the major histocompatibility complex (MHC) class I promoter: identification of a negative regulatory element responsive to Ad12 E1A. Nucleic Acids Res. 1994 Nov 11;22(22):4779–4788. doi: 10.1093/nar/22.22.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Rahal J. J., Millian S. J., Noriega E. R. Coxsackievirus and adenovirus infection. Association with acute febrile and juvenile rheumatoid arthritis. JAMA. 1976 Jun 7;235(23):2496–2501. doi: 10.1001/jama.235.23.2496. [DOI] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. G., Gall E. P., Minnich L. L., Roediger J., De Benedetti C., Corrigan J. J. Acute polyarthritis associated with active Epstein-Barr virus infection. JAMA. 1982 Dec 10;248(22):2990–2993. [PubMed] [Google Scholar]
- Ray P., Black S., Shinefield H., Dillon A., Schwalbe J., Holmes S., Hadler S., Chen R., Cochi S., Wassilak S. Risk of chronic arthropathy among women after rubella vaccination. Vaccine Safety Datalink Team. JAMA. 1997 Aug 20;278(7):551–556. [PubMed] [Google Scholar]
- Rich S. A. Human lupus inclusions and interferon. Science. 1981 Aug 14;213(4509):772–775. doi: 10.1126/science.6166984. [DOI] [PubMed] [Google Scholar]
- Rothschild B. M., Woods R. J., Rothschild C., Sebes J. I. Geographic distribution of rheumatoid arthritis in ancient North America: implications for pathogenesis. Semin Arthritis Rheum. 1992 Dec;22(3):181–187. doi: 10.1016/0049-0172(92)90018-9. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Poggi A., Sitia R., Zocchi M. R. HIV-I Tat: a polypeptide for all seasons. Immunol Today. 1998 Dec;19(12):543–545. doi: 10.1016/s0167-5699(98)01351-6. [DOI] [PubMed] [Google Scholar]
- Ruegg C. L., Strand M. A synthetic peptide with sequence identity to the transmembrane protein GP41 of HIV-1 inhibits distinct lymphocyte activation pathways dependent on protein kinase C and intracellular calcium influx. Cell Immunol. 1991 Oct 1;137(1):1–13. doi: 10.1016/0008-8749(91)90051-c. [DOI] [PubMed] [Google Scholar]
- SIGURDSSON B., PALSSON P., GRIMSSON H. Visna, a demyelinating transmissible disease of sheep. J Neuropathol Exp Neurol. 1957 Jul;16(3):389–403. doi: 10.1097/00005072-195707000-00010. [DOI] [PubMed] [Google Scholar]
- Sabbatini A., Bombardieri S., Migliorini P. Autoantibodies from patients with systemic lupus erythematosus bind a shared sequence of SmD and Epstein-Barr virus-encoded nuclear antigen EBNA I. Eur J Immunol. 1993 May;23(5):1146–1152. doi: 10.1002/eji.1830230525. [DOI] [PubMed] [Google Scholar]
- Salvesen G. S., Dixit V. M. Caspases: intracellular signaling by proteolysis. Cell. 1997 Nov 14;91(4):443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- Sela M. Antigenicity: some molecular aspects. Science. 1969 Dec 12;166(3911):1365–1374. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- Stebbings S., Highton J., Croxson M. C., Powell K., McKay J., Rietveld J. Chickenpox monoarthritis: demonstration of varicella-zoster virus in joint fluid by polymerase chain reaction. Br J Rheumatol. 1998 Mar;37(3):311–313. doi: 10.1093/rheumatology/37.3.311. [DOI] [PubMed] [Google Scholar]
- Steinberg A. D., Gourley M. F., Klinman D. M., Tsokos G. C., Scott D. E., Krieg A. M. NIH conference. Systemic lupus erythematosus. Ann Intern Med. 1991 Oct 1;115(7):548–559. doi: 10.7326/0003-4819-115-7-548. [DOI] [PubMed] [Google Scholar]
- Strack P. R., Frey M. W., Rizzo C. J., Cordova B., George H. J., Meade R., Ho S. P., Corman J., Tritch R., Korant B. D. Apoptosis mediated by HIV protease is preceded by cleavage of Bcl-2. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9571–9576. doi: 10.1073/pnas.93.18.9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stransky G., Vernon J., Aicher W. K., Moreland L. W., Gay R. E., Gay S. Virus-like particles in synovial fluids from patients with rheumatoid arthritis. Br J Rheumatol. 1993 Dec;32(12):1044–1048. doi: 10.1093/rheumatology/32.12.1044. [DOI] [PubMed] [Google Scholar]
- Talal N., Garry R. F., Schur P. H., Alexander S., Dauphinée M. J., Livas I. H., Ballester A., Takei M., Dang H. A conserved idiotype and antibodies to retroviral proteins in systemic lupus erythematosus. J Clin Invest. 1990 Jun;85(6):1866–1871. doi: 10.1172/JCI114647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J. L., Schröter M. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997 Apr 3;386(6624):517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- Tingle A. J., Mitchell L. A., Grace M., Middleton P., Mathias R., MacWilliam L., Chalmers A. Randomised double-blind placebo-controlled study on adverse effects of rubella immunisation in seronegative women. Lancet. 1997 May 3;349(9061):1277–1281. doi: 10.1016/S0140-6736(96)12031-6. [DOI] [PubMed] [Google Scholar]
- Tsao B. P., Cantor R. M., Kalunian K. C., Chen C. J., Badsha H., Singh R., Wallace D. J., Kitridou R. C., Chen S. L., Shen N. Evidence for linkage of a candidate chromosome 1 region to human systemic lupus erythematosus. J Clin Invest. 1997 Feb 15;99(4):725–731. doi: 10.1172/JCI119217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Macen J. L., Schreiber M., McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991 Sep;184(1):370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- Vaughan J. H., Nguyen M. D., Valbracht J. R., Patrick K., Rhodes G. H. Epstein-Barr virus-induced autoimmune responses. II. Immunoglobulin G autoantibodies to mimicking and nonmimicking epitopes. Presence in autoimmune disease. J Clin Invest. 1995 Mar;95(3):1316–1327. doi: 10.1172/JCI117782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. W., Gibson M. K., Vermeulen W., Yeh H., Forrester K., Stürzbecher H. W., Hoeijmakers J. H., Harris C. C. Abrogation of p53-induced apoptosis by the hepatitis B virus X gene. Cancer Res. 1995 Dec 15;55(24):6012–6016. [PubMed] [Google Scholar]
- Weitzul S., Duvic M. HIV-related psoriasis and Reiter's syndrome. Semin Cutan Med Surg. 1997 Sep;16(3):213–218. doi: 10.1016/s1085-5629(97)80044-2. [DOI] [PubMed] [Google Scholar]
- Westendorp M. O., Frank R., Ochsenbauer C., Stricker K., Dhein J., Walczak H., Debatin K. M., Krammer P. H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995 Jun 8;375(6531):497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Yamada T., Yamaoka S., Goto T., Nakai M., Tsujimoto Y., Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994 May;68(5):3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Mellors R. C., Strand M., August J. T. The viral envelope glycoprotein of murine leukemia virus and the pathogenesis of immune complex glomerulonephritis of New Zealand mice. J Exp Med. 1974 Oct 1;140(4):1011–1027. doi: 10.1084/jem.140.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. B., Hsieh S. L., Bentley D. R., Campbell R. D., Volanakis J. E. A variable number of tandem repeats locus within the human complement C2 gene is associated with a retroposon derived from a human endogenous retrovirus. J Exp Med. 1992 Jun 1;175(6):1783–1787. doi: 10.1084/jem.175.6.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]