Abstract
OBJECTIVE—To investigate whether identical T cell clonotypes accumulate in multiple rheumatoid joints, the clonality of T cells that had infiltrated into synovial tissue (ST) samples simultaneously obtained from multiple joints of patients with rheumatoid arthritis (RA) was analysed. METHODS—T cell receptor (TCR) β gene transcripts, amplified by reverse transcription-polymerase chain reaction from ST and peripheral blood lymphocytes of five RA patients, were subjected to single strand conformation polymorphism analysis and DNA sequencing. RESULTS—Approximately 40% of accumulated T cell clonotypes found in one joint of a patient were found in multiple joints in the same patient. Furthermore, identical amino acid sequences were found in TCR β junctional regions of these clonotypes from different patients with at least one HLA molecule match. CONCLUSIONS—The T cell clonotypes accumulating in multiple rheumatoid joints may be involved in the perpetuation of polyarthritis by reacting to antigens common to these multiple joints.
Full Text
The Full Text of this article is available as a PDF (1.4 MB).
Figure 1 .
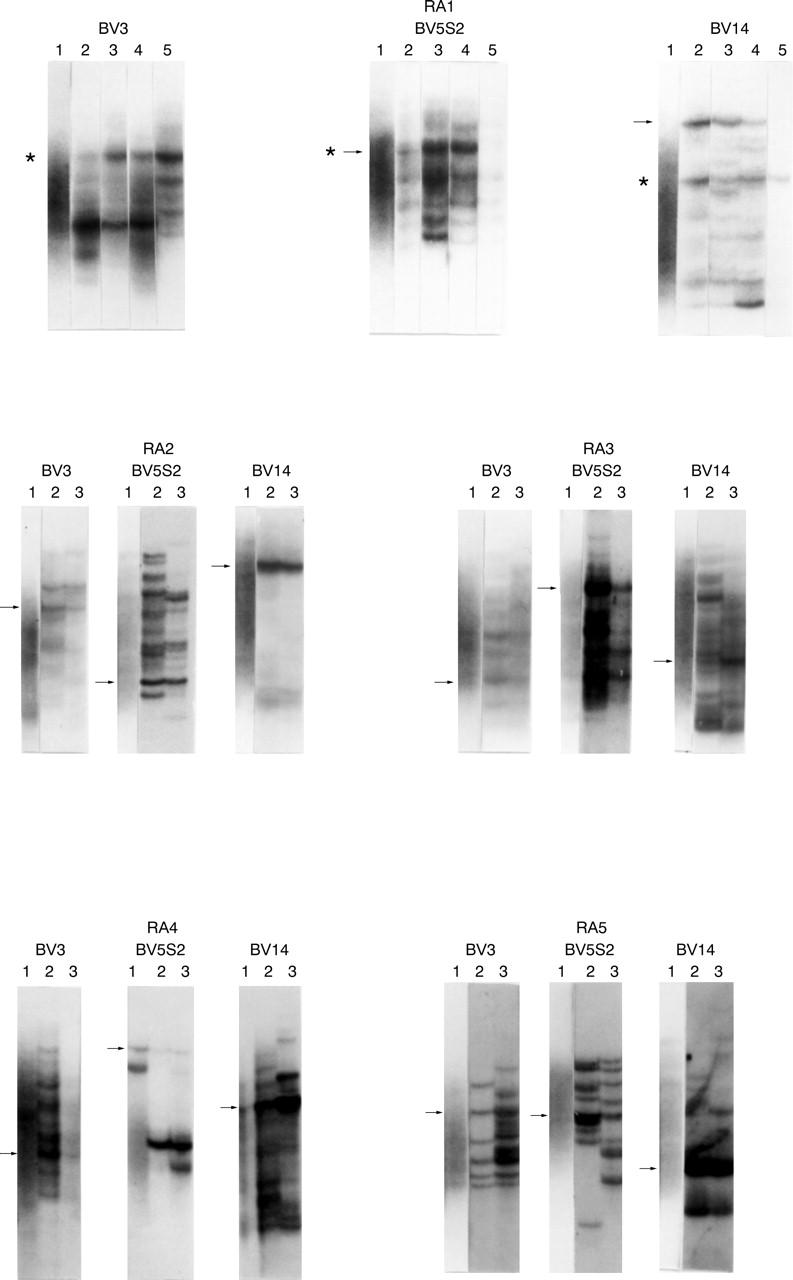
Comparison of accumulated TCR β gene clonotypes in different joints by RT-PCR/SSCP analysis. ST specimens were derived from four different joints of RA1 and two different joints of RA2-RA5, respectively. TCR β gene transcripts, amplified from each specimen by RT-PCR, were separated based on their single strand conformation polymorphism on a single gel to detect TCR β gene bands with identical migrating positions. Some of the distinct TCR β gene bands derived from different specimens were found to migrate to identical positions (indicated by asterisks). This indicates that T cells that possessed TCR β gene transcripts corresponding to these bands accumulated clonally in multiple joints. Results of representative three BV families (BV3, BV5S2 and BV14) are shown. Arrows indicate bands from which TCR β gene transcripts were recovered for determination of nucleotide sequences. The results of RA1 are shown as follows: (lane 1, PBLs; lane 2, ST from the left ankle; lane 3, ST from the right ankle; lane 4, ST from the left PIP; and lane 5, ST from the left elbow). The results of RA2-RA5 are shown as follows: RA2: lane 1, PBL; lane 2, ST from the right MP; lane 3, ST from the right knee. Similarly, RA3: lane 1, PBL; lane 2, ST from the right MP; lane 3, ST from the right elbow. RA4: lane 1, PBL; lane 2, ST from the right wrist; lane 3, ST from the left knee. RA5: lane 1, PBL; lane 2, ST from the right knee; lane 3, ST from the left knee.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam A., Lambert N., Lulé J., Coppin H., Mazières B., de Préval C., Cantagrel A. Persistence of dominant T cell clones in synovial tissues during rheumatoid arthritis. J Immunol. 1996 May 1;156(9):3480–3485. [PubMed] [Google Scholar]
- Alam A., Lulé J., Coppin H., Lambert N., Maziéres B., De Préval C., Cantagrel A. T-cell receptor variable region of the beta-chain gene use in peripheral blood and multiple synovial membranes during rheumatoid arthritis. Hum Immunol. 1995 Apr;42(4):331–339. doi: 10.1016/0198-8859(94)00121-6. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bhayani H., Paterson Y. Analysis of peptide binding patterns in different major histocompatibility complex/T cell receptor complexes using pigeon cytochrome c-specific T cell hybridomas. Evidence that a single peptide binds major histocompatibility complex in different conformations. J Exp Med. 1989 Nov 1;170(5):1609–1625. doi: 10.1084/jem.170.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerasoli D. M., Riley M. P., Shih F. F., Caton A. J. Genetic basis for T cell recognition of a major histocompatibility complex class II-restricted neo-self peptide. J Exp Med. 1995 Nov 1;182(5):1327–1336. doi: 10.1084/jem.182.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. M., Roessner K. D., Naito-Hoopes M., Howard D. B., Gaur L. K., Budd R. C. Increased usage of V beta 2 and V beta 6 in rheumatoid synovial fluid T cells. Arthritis Rheum. 1994 Nov;37(11):1627–1636. doi: 10.1002/art.1780371112. [DOI] [PubMed] [Google Scholar]
- Dessen A., Lawrence C. M., Cupo S., Zaller D. M., Wiley D. C. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity. 1997 Oct;7(4):473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. E., Ricalton N. S., Meyer A. C., West S. G., Kaplan H., Behrendt C., Kotzin B. L. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995 Apr 1;154(7):3538–3547. [PubMed] [Google Scholar]
- Garboczi D. N., Ghosh P., Utz U., Fan Q. R., Biddison W. E., Wiley D. C. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996 Nov 14;384(6605):134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- Garcia K. C., Degano M., Stanfield R. L., Brunmark A., Jackson M. R., Peterson P. A., Teyton L., Wilson I. A. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996 Oct 11;274(5285):209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- González-Quintial R., Baccalá R., Pope R. M., Theofilopoulos A. N. Identification of clonally expanded T cells in rheumatoid arthritis using a sequence enrichment nuclease assay. J Clin Invest. 1996 Mar 1;97(5):1335–1343. doi: 10.1172/JCI118550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J. J., Bartz-Bazzanella P., Hu W., Jendro M. C., Walser-Kuntz D. R., Weyand C. M. Dominant clonotypes in the repertoire of peripheral CD4+ T cells in rheumatoid arthritis. J Clin Invest. 1994 Nov;94(5):2068–2076. doi: 10.1172/JCI117561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grom A. A., Thompson S. D., Luyrink L., Passo M., Choi E., Glass D. N. Dominant T-cell-receptor beta chain variable region V beta 14+ clones in juvenile rheumatoid arthritis. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11104–11108. doi: 10.1073/pnas.90.23.11104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell M. D., Diveley J. P., Lundeen K. A., Esty A., Winters S. T., Carlo D. J., Brostoff S. W. Limited T-cell receptor beta-chain heterogeneity among interleukin 2 receptor-positive synovial T cells suggests a role for superantigen in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10921–10925. doi: 10.1073/pnas.88.23.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Masuko K., Nakai Y., Kato T., Hasanuma T., Yoshino S. I., Mizushima Y., Nishioka K., Yamamoto K. High frequencies of identical T cell clonotypes in synovial tissues of rheumatoid arthritis patients suggest the occurrence of common antigen-driven immune responses. Arthritis Rheum. 1996 Mar;39(3):446–453. doi: 10.1002/art.1780390312. [DOI] [PubMed] [Google Scholar]
- Jenkins R. N., Nikaein A., Zimmermann A., Meek K., Lipsky P. E. T cell receptor V beta gene bias in rheumatoid arthritis. J Clin Invest. 1993 Dec;92(6):2688–2701. doi: 10.1172/JCI116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Ikeda Y., Zong Z. P., Sasakawa H., Kurokawa M., Masuko K., Igarashi R., Mizushima Y., Nishioka K., Yamamoto K. Characterization of T cell receptor beta chains of accumulating T cells in skin allografts in mice. Transplantation. 1996 Jul 27;62(2):266–272. doi: 10.1097/00007890-199607270-00020. [DOI] [PubMed] [Google Scholar]
- Khazaei H. A., Lunardi C., So A. K. CD4 T cells in the rheumatoid joint are oligoclonally activated and change during the course of disease. Ann Rheum Dis. 1995 Apr;54(4):314–317. doi: 10.1136/ard.54.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawinkel U., Pluschke G. T cell receptor variable region repertoire in lymphocytes from rheumatoid arthritis patients. Immunobiology. 1992 Sep;185(5):483–491. doi: 10.1016/S0171-2985(11)80090-2. [DOI] [PubMed] [Google Scholar]
- Li Y., Sun G. R., Tumang J. R., Crow M. K., Friedman S. M. CDR3 sequence motifs shared by oligoclonal rheumatoid arthritis synovial T cells. Evidence for an antigen-driven response. J Clin Invest. 1994 Dec;94(6):2525–2531. doi: 10.1172/JCI117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunardi C., Marguerie C., So A. K. An altered repertoire of T cell receptor V gene expression by rheumatoid synovial fluid T lymphocytes. Clin Exp Immunol. 1992 Dec;90(3):440–446. doi: 10.1111/j.1365-2249.1992.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T., Saito I., Miyake S., Hashimoto H., Sato K., Yagita H., Okumura K., Miyasaka N. A possible role of two hydrophobic amino acids in antigen recognition by synovial T cells in rheumatoid arthritis. Eur J Immunol. 1993 Sep;23(9):2059–2065. doi: 10.1002/eji.1830230903. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Byers P., Seyfried C., Healey L. A., Wilske K. R., Stage D., Nepom B. S. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989 Jan;32(1):15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- Okubo M., Kurokawa M., Ohto H., Nishimaki T., Nishioka K., Kasukawa R., Yamamoto K. Clonotype analysis of peripheral blood T cells and autoantigen-reactive T cells from patients with mixed connective tissue disease. J Immunol. 1994 Oct 15;153(8):3784–3790. [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Pluschke G., Ginter A., Taube H., Melchers I., Peter H. H., Krawinkel U. Analysis of T cell receptor V beta regions expressed by rheumatoid synovial T lymphocytes. Immunobiology. 1993 Aug;188(4-5):330–339. doi: 10.1016/s0171-2985(11)80217-2. [DOI] [PubMed] [Google Scholar]
- Rowen L., Koop B. F., Hood L. The complete 685-kilobase DNA sequence of the human beta T cell receptor locus. Science. 1996 Jun 21;272(5269):1755–1762. doi: 10.1126/science.272.5269.1755. [DOI] [PubMed] [Google Scholar]
- Sasazuki T., Kaneoka H., Ohta N., Hayase R., Iwamoto I. Four common HLA haplotypes and their association with diseases in the Japanese population. Transplant Proc. 1979 Dec;11(4):1871–1873. [PubMed] [Google Scholar]
- Sottini A., Imberti L., Bettinardi A., Mazza C., Gorla R., Primi D. Selection of T lymphocytes in two rheumatoid arthritis patients defines different T-cell receptor V beta repertoires in CD4+ and CD8+ T-cell subsets. J Autoimmun. 1993 Oct;6(5):621–637. doi: 10.1006/jaut.1993.1051. [DOI] [PubMed] [Google Scholar]
- Struyk L., Hawes G. E., Dolhain R. J., van Scherpenzeel A., Godthelp B., Breedveld F. C., van den Elsen P. J. Evidence for selective in vivo expansion of synovial tissue-infiltrating CD4+ CD45RO+ T lymphocytes on the basis of CDR3 diversity. Int Immunol. 1994 Jun;6(6):897–907. doi: 10.1093/intimm/6.6.897. [DOI] [PubMed] [Google Scholar]
- Todd J. A., Acha-Orbea H., Bell J. I., Chao N., Fronek Z., Jacob C. O., McDermott M., Sinha A. A., Timmerman L., Steinman L. A molecular basis for MHC class II--associated autoimmunity. Science. 1988 May 20;240(4855):1003–1009. doi: 10.1126/science.3368786. [DOI] [PubMed] [Google Scholar]
- Uematsu Y., Wege H., Straus A., Ott M., Bannwarth W., Lanchbury J., Panayi G., Steinmetz M. The T-cell-receptor repertoire in the synovial fluid of a patient with rheumatoid arthritis is polyclonal. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakitani S., Murata N., Toda Y., Ogawa R., Kaneshige T., Nishimura Y., Ochi T. The relationship between HLA-DRB1 alleles and disease subsets of rheumatoid arthritis in Japanese. Br J Rheumatol. 1997 Jun;36(6):630–636. doi: 10.1093/rheumatology/36.6.630. [DOI] [PubMed] [Google Scholar]
- Walser-Kuntz D. R., Weyand C. M., Weaver A. J., O'Fallon W. M., Goronzy J. J. Mechanisms underlying the formation of the T cell receptor repertoire in rheumatoid arthritis. Immunity. 1995 Jun;2(6):597–605. doi: 10.1016/1074-7613(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Williams W. V., Kieber-Emmons T., Fang Q., Von Feldt J., Wang B., Ramanujam T., Weiner D. B. Conserved motifs in rheumatoid arthritis synovial tissue T-cell receptor beta chains. DNA Cell Biol. 1993 Jun;12(5):425–434. doi: 10.1089/dna.1993.12.425. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Masuko K., Takahashi S., Ikeda Y., Kato T., Mizushima Y., Hayashi K., Nishioka K. Accumulation of distinct T cell clonotypes in human solid tumors. J Immunol. 1995 Feb 15;154(4):1804–1809. [PubMed] [Google Scholar]
- Yamamoto K., Sakoda H., Nakajima T., Kato T., Okubo M., Dohi M., Mizushima Y., Ito K., Nishioka K. Accumulation of multiple T cell clonotypes in the synovial lesions of patients with rheumatoid arthritis revealed by a novel clonality analysis. Int Immunol. 1992 Nov;4(11):1219–1223. doi: 10.1093/intimm/4.11.1219. [DOI] [PubMed] [Google Scholar]


