Abstract
OBJECTIVE—To investigate endothelial cell adhesion molecule expression and leucocyte adhesion to endothelial cells isolated from the microvasculature of rheumatoid arthritic synovial tissue (SMEC) in comparison with similar cells isolated from healthy subcutaneous adipose tissue (ADMEC) or from umbilical veins (HUVEC). METHODS—Cultured endothelial cells were treated with tumour necrosis factor α (TNFα) for 2-24 hours before the assessment of cell surface E-selectin, vascular (VCAM-1) or intercellular cell adhesion molecule-I (ICAM-1) expression. Neutrophil and T lymphocyte adhesion to TNFα treated endothelial cells was assessed using static and shear dependent assay systems. RESULTS—VCAM-1 expression by SMEC was significantly less sensitive to TNFα stimulation than HUVEC or ADMEC. E-selectin expression by SMEC appeared to be more sensitive to TNFα stimulation and maximal expression was about 30% greater in comparison with HUVEC or ADMEC. Sensitivity to TNFα induction and maximal ICAM-1 expression was similar in all three endothelial cell types. Static neutrophil adhesion to TNFα stimulated SMEC was significantly increased in comparison with HUVEC, however this phenomenon was dependent on the presence of neutralising antibodies to ICAM-1. At shear rates in excess of 2.4 dynes/cm2 significantly more neutrophils and, predominantly CD45RO+, T lymphocytes adhered to TNFα stimulated SMEC than HUVEC. CONCLUSION—Rheumatoid synovial endothelial cells differentially regulate E-selectin and VCAM-1. The increased ability of TNFα stimulated synovial endothelial cells to support leucocyte adhesion may help to explain the leucocyte, in particular CD45RO+ T-lymphocyte, recruitment observed in the rheumatoid synovium.
Full Text
The Full Text of this article is available as a PDF (205.7 KB).
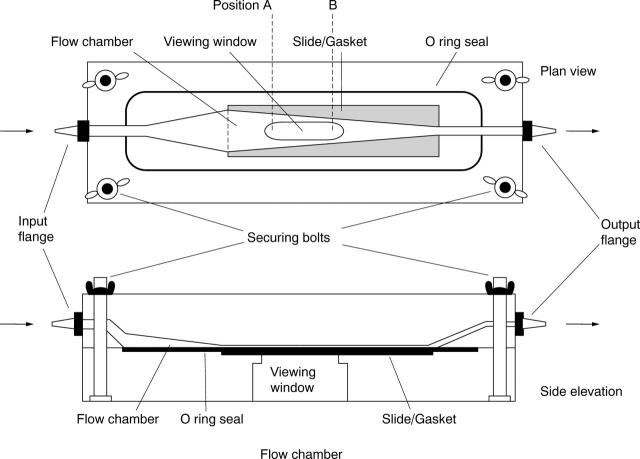
Figure 1 .
Schematic representation of the parallel plate flow cell used. The convergence of the side walls of the cell increases the velocity of the perfusate as it travels through the cell. This increase in velocity permits measurements to be made on cells exposed to an increasing wall shear stress in the same system without changing the mass flow volume. Wall shear stress at position A (τA) = 2.4 dynes/cm2 and τB = 4.0 dynes/cm2 assuming a volumetric flow rate of 0.916 cm-3/sec (55 ml/min).
Figure 2 .
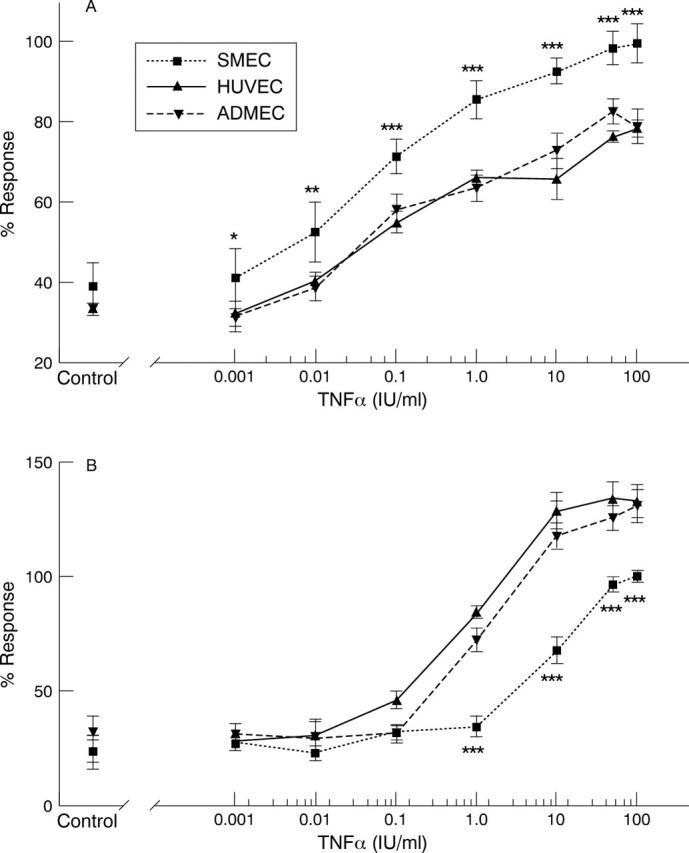
(A) HUVEC, ADMEC and SMEC monolayers were stimulated TNFα (0-100 IU/ml) for six hours before assessment of E-selectin expression. Results are normalised as percentages of the optical density observed in SMEC cultures stimulated with 100 IU/ml TNFα and depict 95% confidence intervals for the mean response. E-selectin expression by SMEC was compared with both HUVEC and ADMEC. SMEC display significantly (p<0.05*, p<0.01**, p<0.001***) higher E-selectin expression than HUVEC or ADMEC at all concentrations of TNFα, (n=3). (B) HUVEC, ADMEC and SMEC monolayers were pre-incubated with TNFα (0-100 IU/ml) for six hours before assessment of VCAM-1 expression. Results are normalised as percentages of the optical density observed in SMEC cultures stimulated with 100 IU/ml TNFα and depict 95% confidence intervals for the mean response. VCAM-1 expression by HUVEC and ADMEC was not significantly different. SMEC display significantly (p<0.001***) lower VCAM-1 expression than HUVEC or ADMEC at concentrations of TNFα in excess of 1 IU/ml, (n=3).
Figure 3 .
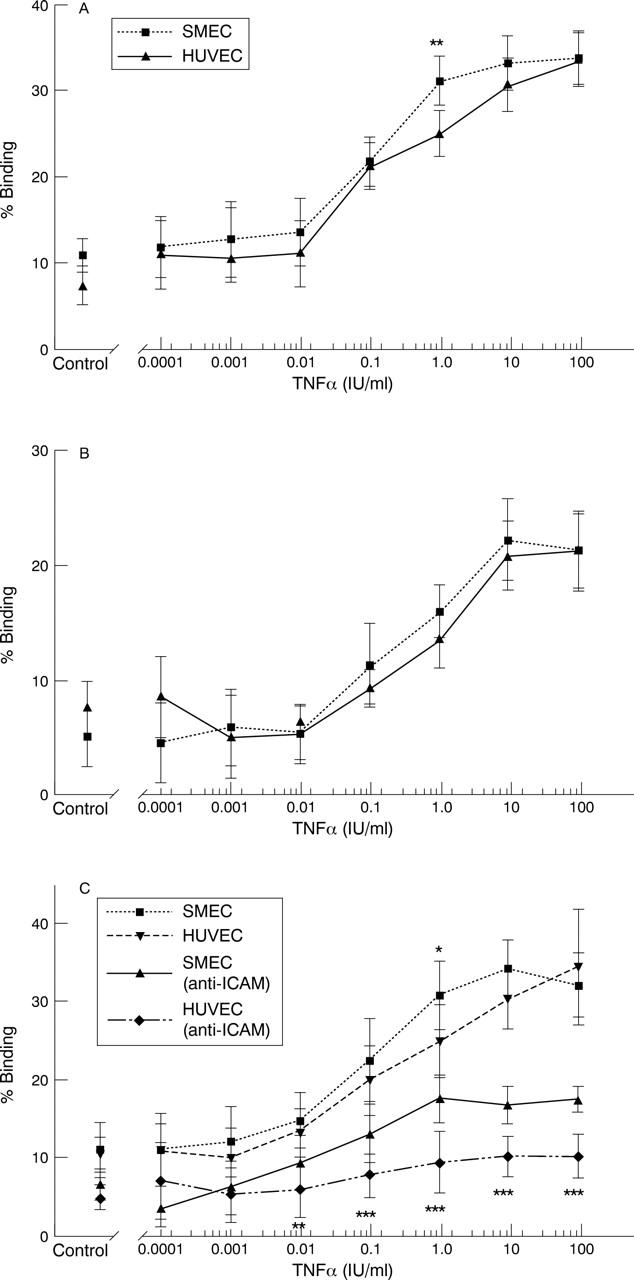
(A) Neutrophil adhesion to HUVEC and SMEC monolayers pre-incubated with TNFα (0-100 IU/ml). Results show mean binding of neutrophils as a percentage of the initial number added and depict 95% confidence intervals for the mean binding. SMEC occasionally, but not consistently, bind significantly (p<0.01**) higher numbers of neutrophils than HUVEC (n=5). (B) T lymphocyte adhesion to HUVEC and SMEC monolayers pre-incubated with TNFα (0-100 IU/ml). Results are expressed as the mean percentage binding of the original number of lymphocytes added and depict 95% confidence intervals for the mean binding. No significant differences were observed between cell types (n=5). (C) Comparison of static PMN adhesion to TNFα (0-100 IU/ml) stimulated endothelial cell monolayers. Results show mean binding of neutrophils as a percentage of the initial number added and depict 95% confidence intervals for the mean binding. Results indicate differences in neutrophil binding to the two endothelial cell types before the addition of anti-ICAM-1 antibody (50 µg/ml), rarely reaches significance (p<0.05*). However, in the presence of anti-ICAM-1 antibody SMEC are capable of binding significantly (p<0.05*, p<0.01**, p<0.001***) more PMN than similarly treated HUVEC at a number of TNFα concentrations (n=3).
Figure 4 .
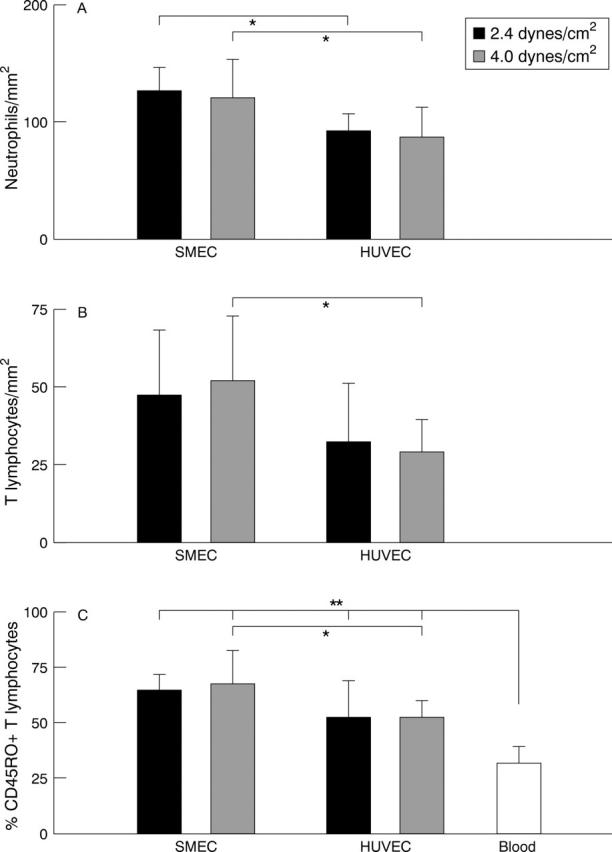
Adhesion of (A) peripheral blood neutrophils and (B) T lymphocytes to SMEC and HUVEC monolayers pre-stimulated with TNFα (50 IU/ml) for five hours. The cell counts represents the mean number of leucocytes (per mm2) attached to the endothelial monolayers, after 30 minutes of continuous circulation, at points in the flow chamber where wall shear rates were calculated at 2.4 and 4.0 dynes/cm2 respectively and depict 95% confidence intervals for the mean response. SMEC monolayers bound significantly (p<0.05*) higher numbers of both neutrophils at both 2.4 and 4.0 dynes/cm2 in comparison with HUVEC at the same rates of shear. Differences in T lymphocytes adhesion to SMEC in comparison with HUVEC were only significant (p<0.05*) at higher (4.0 dynes/cm2) rates of shear (n=3). (C) Immunohistochemical analysis of adherent T lymphocyte phenotype and initial proportions in healthy peripheral blood. Results are expressed as the proportion of cells bearing a CD45RO+ phenotype as a percentage of the total and depict 95% confidence intervals for the mean response. Cell populations adherent to both SMEC and HUVEC monolayers at both 2.4 and 4.0 dynes/cm2 display a significant (p<0.01**) increase in the proportions of CD45RO+ cells in comparison with peripheral blood. SMEC also display a significantly (p<0.05*) increased capacity to bind such cells in comparison to HUVEC at higher (4.0 dynes/cm2) rates of shear (n=3).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbot S. E., Kaul A., Stevens C. R., Blake D. R. Isolation and culture of synovial microvascular endothelial cells. Characterization and assessment of adhesion molecule expression. Arthritis Rheum. 1992 Apr;35(4):401–406. doi: 10.1002/art.1780350407. [DOI] [PubMed] [Google Scholar]
- Alon R., Kassner P. D., Carr M. W., Finger E. B., Hemler M. E., Springer T. A. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995 Mar;128(6):1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E. L., McEvoy L. M., Berlin C., Bargatze R. F., Butcher E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993 Dec 16;366(6456):695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Chen X. L., Tummala P. E., Olliff L., Medford R. M. E-selectin gene expression in vascular smooth muscle cells. Evidence for a tissue-specific repressor protein. Circ Res. 1997 Mar;80(3):305–311. doi: 10.1161/01.res.80.3.305. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke O. L., Hobbs S., Panayi G. S., Poulter L. W., Rasker J. J., Janossy G. A combined immunohistological and histochemical analysis of lymphocyte and macrophage subpopulations in the rheumatoid nodule. Clin Exp Immunol. 1984 May;56(2):239–246. [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Tsai V., Strahl D., Goel A. S., Tollefson V., Arrhenius T., Wayner E. A., Gaeta F. C., Fikes J. D., Firestein G. S. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994 Jan;93(1):405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Kieffer J. D., Armerding D., Kupper T. S. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997 Oct 30;389(6654):978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Graber N., Gopal T. V., Wilson D., Beall L. D., Polte T., Newman W. T cells bind to cytokine-activated endothelial cells via a novel, inducible sialoglycoprotein and endothelial leukocyte adhesion molecule-1. J Immunol. 1990 Aug 1;145(3):819–830. [PubMed] [Google Scholar]
- Haskard D. O., Cavender D., Fleck R. M., Sontheimer R., Ziff M. Human dermal microvascular endothelial cells behave like umbilical vein endothelial cells in T-cell adhesion studies. J Invest Dermatol. 1987 Mar;88(3):340–344. doi: 10.1111/1523-1747.ep12466229. [DOI] [PubMed] [Google Scholar]
- Iademarco M. F., Barks J. L., Dean D. C. Regulation of vascular cell adhesion molecule-1 expression by IL-4 and TNF-alpha in cultured endothelial cells. J Clin Invest. 1995 Jan;95(1):264–271. doi: 10.1172/JCI117650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. A., McIntire L. V., Smith C. W., Picker L. J. A two-step adhesion cascade for T cell/endothelial cell interactions under flow conditions. J Clin Invest. 1994 Dec;94(6):2443–2450. doi: 10.1172/JCI117612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. M., Watts G. M., Robinson M. K., Vestweber D., Jutila M. A. Comparison of E-selectin-binding glycoprotein ligands on human lymphocytes, neutrophils, and bovine gamma delta T cells. J Immunol. 1997 Oct 1;159(7):3574–3583. [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of the cartilage-pannus junction in rheumatoid arthritis. Arthritis Rheum. 1975 Sep-Oct;18(5):475–483. doi: 10.1002/art.1780180507. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Burrows J. C., Haines G. K., Carlos T. M., Harlan J. M., Leibovich S. J. Immunolocalization of endothelial and leukocyte adhesion molecules in human rheumatoid and osteoarthritic synovial tissues. Lab Invest. 1991 Mar;64(3):313–320. [PubMed] [Google Scholar]
- Lo S. K., Lee S., Ramos R. A., Lobb R., Rosa M., Chi-Rosso G., Wright S. D. Endothelial-leukocyte adhesion molecule 1 stimulates the adhesive activity of leukocyte integrin CR3 (CD11b/CD18, Mac-1, alpha m beta 2) on human neutrophils. J Exp Med. 1991 Jun 1;173(6):1493–1500. doi: 10.1084/jem.173.6.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marui N., Offermann M. K., Swerlick R., Kunsch C., Rosen C. A., Ahmad M., Alexander R. W., Medford R. M. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 1993 Oct;92(4):1866–1874. doi: 10.1172/JCI116778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P. G., Orkin R. W. Isolation of rat aortic endothelial cells by primary explant techniques and their phenotypic modulation by defined substrata. Lab Invest. 1987 Jul;57(1):94–105. [PubMed] [Google Scholar]
- Montgomery K. F., Osborn L., Hession C., Tizard R., Goff D., Vassallo C., Tarr P. I., Bomsztyk K., Lobb R., Harlan J. M. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6523–6527. doi: 10.1073/pnas.88.15.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Pober J. S., Cotran R. S. Recruitment of neutrophils in the local endotoxin response: association with de novo endothelial expression of endothelial leukocyte adhesion molecule-1. Lab Invest. 1991 Feb;64(2):295–299. [PubMed] [Google Scholar]
- Neish A. S., Read M. A., Thanos D., Pine R., Maniatis T., Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 1995 May;15(5):2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris P., Poston R. N., Thomas D. S., Thornhill M., Hawk J., Haskard D. O. The expression of endothelial leukocyte adhesion molecule-1 (ELAM-1), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) in experimental cutaneous inflammation: a comparison of ultraviolet B erythema and delayed hypersensitivity. J Invest Dermatol. 1991 May;96(5):763–770. doi: 10.1111/1523-1747.ep12471720. [DOI] [PubMed] [Google Scholar]
- Oppenheimer-Marks N., Davis L. S., Bogue D. T., Ramberg J., Lipsky P. E. Differential utilization of ICAM-1 and VCAM-1 during the adhesion and transendothelial migration of human T lymphocytes. J Immunol. 1991 Nov 1;147(9):2913–2921. [PubMed] [Google Scholar]
- Perry M. A., Granger D. N. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J Clin Invest. 1991 May;87(5):1798–1804. doi: 10.1172/JCI115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Kishimoto T. K., Smith C. W., Warnock R. A., Butcher E. C. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991 Feb 28;349(6312):796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- Pitzalis C. The Michael Mason Prize Essay 1996. Role of adhesion mechanisms in the pathogenesis of chronic synovitis. Br J Rheumatol. 1996 Dec;35(12):1198–1215. doi: 10.1093/rheumatology/35.12.1198. [DOI] [PubMed] [Google Scholar]
- Pober J., Cotran R. S. What can be learned from the expression of endothelial adhesion molecules in tissues? Lab Invest. 1991 Mar;64(3):301–305. [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Shaw S., Graber N., Gopal T. V., Horgan K. J., Van Seventer G. A., Newman W. Activation-independent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1991 Feb 28;349(6312):799–802. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- Sriramarao P., von Andrian U. H., Butcher E. C., Bourdon M. A., Broide D. H. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J Immunol. 1994 Nov 1;153(9):4238–4246. [PubMed] [Google Scholar]
- To S. S., Newman P. M., Hyland V. J., Robinson B. G., Schrieber L. Regulation of adhesion molecule expression by human synovial microvascular endothelial cells in vitro. Arthritis Rheum. 1996 Mar;39(3):467–477. doi: 10.1002/art.1780390315. [DOI] [PubMed] [Google Scholar]
- Tonnesen M. G., Anderson D. C., Springer T. A., Knedler A., Avdi N., Henson P. M. Adherence of neutrophils to cultured human microvascular endothelial cells. Stimulation by chemotactic peptides and lipid mediators and dependence upon the Mac-1, LFA-1, p150,95 glycoprotein family. J Clin Invest. 1989 Feb;83(2):637–646. doi: 10.1172/JCI113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldorf H. A., Walsh L. J., Schechter N. M., Murphy G. F. Early cellular events in evolving cutaneous delayed hypersensitivity in humans. Am J Pathol. 1991 Feb;138(2):477–486. [PMC free article] [PubMed] [Google Scholar]
- Wellicome S. M., Kapahi P., Mason J. C., Lebranchu Y., Yarwood H., Haskard D. O. Detection of a circulating form of vascular cell adhesion molecule-1: raised levels in rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 1993 Jun;92(3):412–418. doi: 10.1111/j.1365-2249.1993.tb03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellicome S. M., Thornhill M. H., Pitzalis C., Thomas D. S., Lanchbury J. S., Panayi G. S., Haskard D. O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990 Apr 1;144(7):2558–2565. [PubMed] [Google Scholar]