Abstract
OBJECTIVES—Infectious agents like parvovirus have been implicated as exogenous factors that could trigger onset of systemic lupus erythematosus (SLE). A number of case reports describing a SLE-like presentation of acute human parvovirus B19 infection have been published, but no systematic investigation of the actual seroprevalence in epidemiologically defined SLE populations has previously been reported. METHODS—Sera from 99 SLE patients from a defined area in Southern Sweden, representing 88% of all new SLE cases 1981-1995 within the Lund-Orup Health Care district with 175 000 adult inhabitants (> 15 years of age), and sera from 99 age and sex matched healthy controls were investigated for the presence of IgG parvovirus antibodies. Two different commercially available EIA kits were used; one using E coli synthesised parvovirus VP1/VP2 antigen, and one using baculovirus derived parvovirus VP2 antigen. RESULTS—The EIA using baculovirus derived antigen was more sensitive and surprisingly the controls were more often positive than the SLE patients were (79% versus 65%, χ2 p=0.027). No difference between the groups was seen with the EIA using E coli derived antigen (46% versus 49%). Titration experiments indicated that the discordance between the two tests was a matter of sensitivity rather than specificity. CONCLUSION—No evidence was found of human parvovirus B19 infection being more prevalent among SLE patients. On the contrary, in one of the parvovirus EIAs the controls were more often positive than the SLE patients were.
Full Text
The Full Text of this article is available as a PDF (123.4 KB).
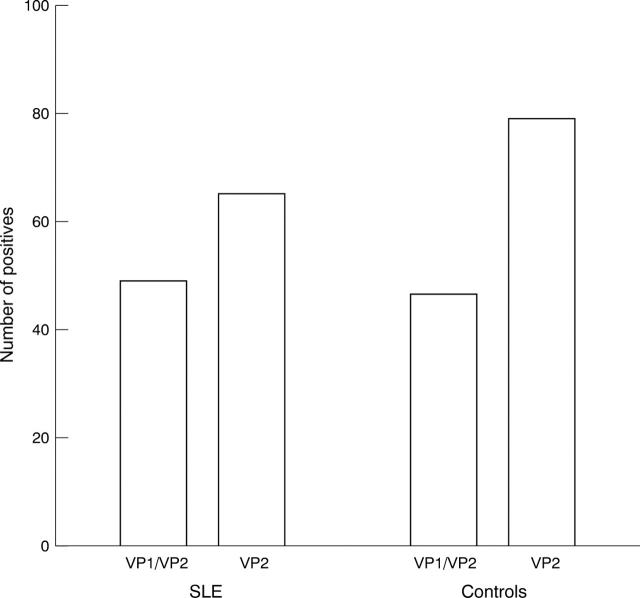
Figure 1 .
Number of positives in the two different parvovirus B19 EIAs. No difference was seen between the 99 SLE patients and the 99 controls with the IgG VP1/VP2 EIA (Progen), 49% of the SLE patients were positive compared with 46% of the controls. The VP2 EIA (Biotrin) was more sensitive and in this case the controls were significantly more often positive than the SLE patients (p = 0.027), 65% of the SLE patients were positive compared with 79% of the controls.