Abstract
OBJECTIVE—To investigate whether quantitative alterations of both β2microglobulin (β2µ) associated HLA class I heavy chains (sHLA-I) and β2 µ free class I heavy chains (sHLA-FHC) in sera of patients with hepatitis C virus (HCV) infection occur and whether they distinguish patients with mixed cryoglobulinaemia (MC). METHODS—83 HCV infected patients were studied and divided into three groups: (A) without cryoglobulinaemia (n=21), (B) with polyclonal MC (n=20), (C) with monoclonal MC (n=42). Serum sHLA-I and sHLA-FHC were measured by double determinant radioimmunoassay using monoclonal antibodies: TP25.99 as catching antibody, and NAMB-1 and HC-10 as revealing antibodies. Western blot identified HLA-I isoforms. RESULTS—The serum concentrations of sHLA-I and of sHLA-FHC in HCV infected patients versus controls were respectively 1.3(0.5) µg/ml (mean (SD)) versus 0.8 (0.3) (p<0.001) and 13.9 (7.1) ng/ml versus 9.2 (5) (p<0.001). sHLA-I were 1.01 (0.4) µg/ml in group A, 1.04 (0.4) µg/ml in group B, and 1.47 (0.4) µg/ml in group C (p=0.001). Statistical analysis showed a significant difference versus controls for groups B (p<0.02) and C (p<0.001). sHLA-FHC were 12.8 (8.3) ng/ml in group A, 17.2 (7.1) ng/ml in group B, and 12.9 (6.2) ng/ml in group C (p<0.02). A significant difference versus controls for each group was found (p<0.02, p<0.001, and p<0.02, respectively). Different patterns of sHLA-I isoforms were observed. CONCLUSIONS—Increased serum concentrations of sHLA-I and sHLA-FHC characterise HCV infected patients. The highest sHLA-I concentrations seem to distinguish patients with monoclonal MC. In this last condition sHLA could play a part in the HCV escape and in B cell proliferation. The significance of sHLA-FHC is still undefined.
Full Text
The Full Text of this article is available as a PDF (205.5 KB).
Figure 1 .
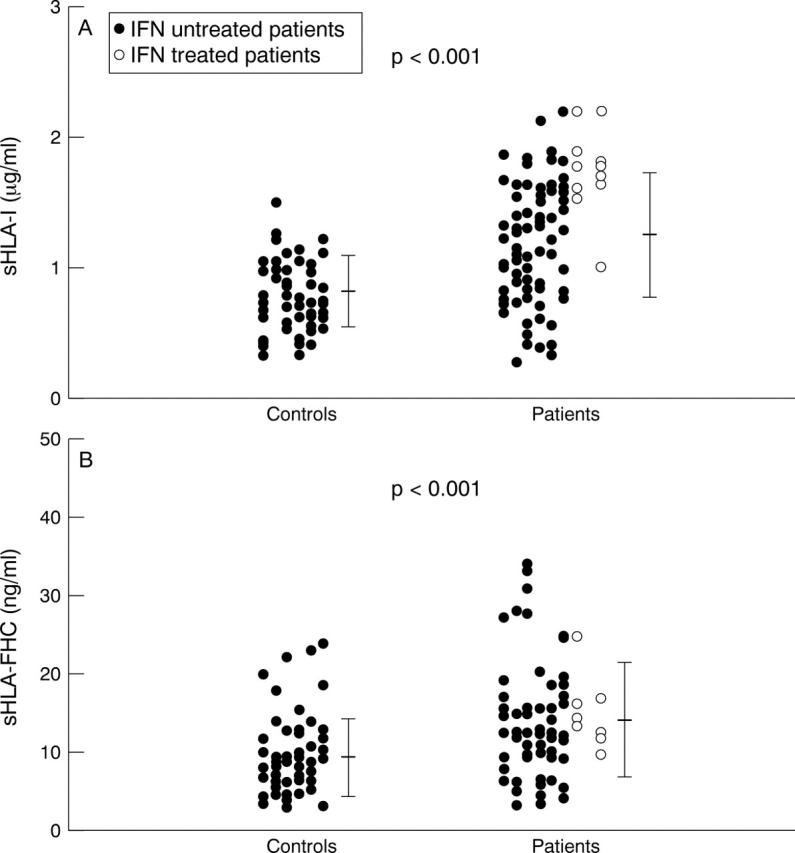
Concentrations of sHLA-I and sHLA-FHC in serum of HCV infected patients and in controls. sHLA-FHC values are expressed in terms of ng/ml of bound 125I-mAb HC-10. Each dot indicates the value of sHLA-I (panel A) and of sHLA-FHC (panel B) in a serum sample. The bars indicate means (SD).
Figure 2 .
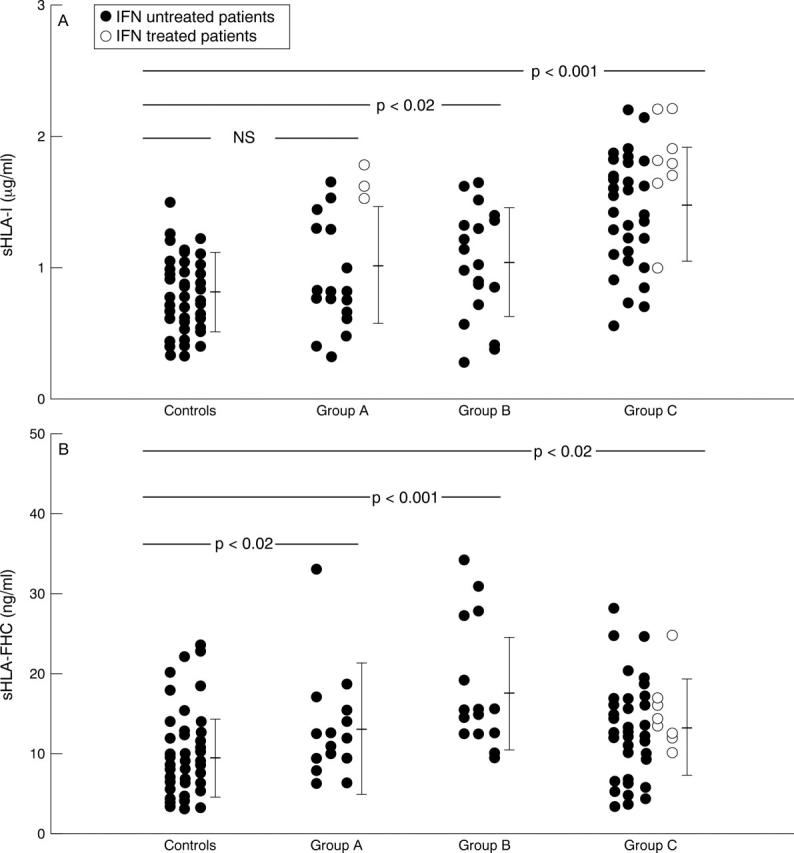
Concentrations of sHLA-I and sHLA-FHC in the serum of HCV infected patients, divided by disease pattern, and in controls. sHLA-FHC values are expressed in terms of ng/ml of bound 125I-mAb HC-10. Each dot indicates the value of sHLA-I (panel A) and of sHLA-FHC (panel B) in a serum sample. The bars indicate means (SD).
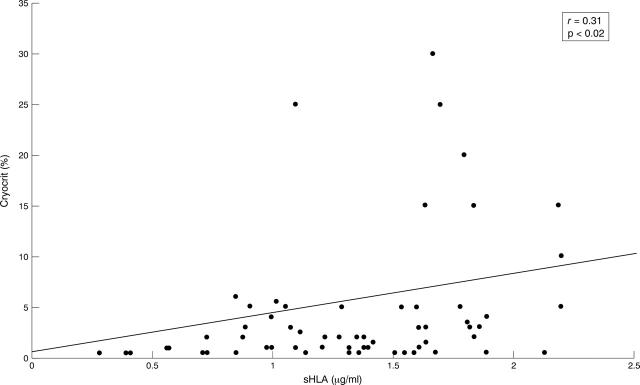
Figure 3 .
Relation between serum sHLA-I concentrations and cryocrit.
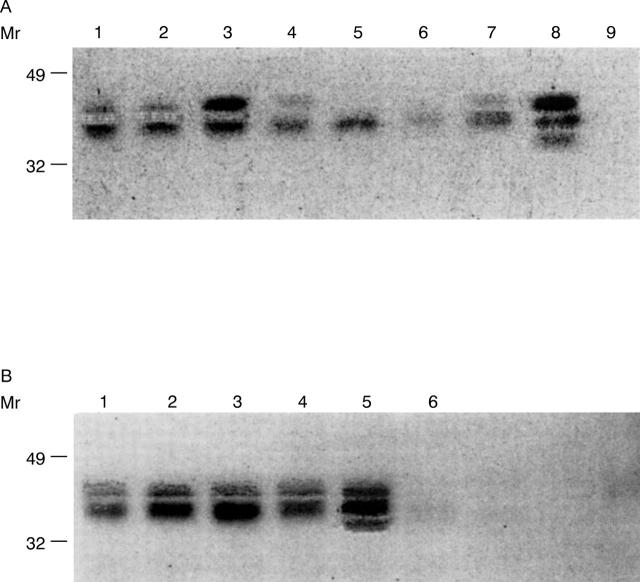
Figure 4 .
Western blot analysis of sHLA-I and sHLA-FHC isolated from sera of HCV infected patients and controls. HLA-I were immunoprecipitated by anti β2-µ mAb NAMB-1 (panel A) from sera of HCV infected patients and controls. Lanes 1 to 3: patients with MC and CS; lanes 4-5: patients with MC without CS; lanes 6-7: patients without MC; lane 8: controls; lane 9: PBS. HLA-FHC were immunoprecipitated by anti-HLA-FHC mAB HC-10 (panel B) from sera of HCV infected patients and controls. Lanes 1-2: patients with MC and CS; lane 3: patient with MC without CS; lane 4: patient without MC; lane 5: controls; lane 6: PBS. After separation by SDS-PAGE, antigens were transferred to a nitrocellulose membrane and detected by 125I-mAb TP25.99. PBS was used as a negative control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnello V. The etiology and pathophysiology of mixed cryoglobulinemia secondary to hepatitis C virus infection. Springer Semin Immunopathol. 1997;19(1):111–129. doi: 10.1007/BF00945029. [DOI] [PubMed] [Google Scholar]
- Alvarez-Cermeño J. C., Casado C., Villar L. M., Ferreira A., Varela J. M., Dominguez M., Bootello A., Najera R., Gonzalez-Porque P. Soluble class 1 antigens (sHLA) in CSF and serum of patients with HIV infection. Acta Neurol Scand. 1990 Jul;82(1):14–16. doi: 10.1111/j.1600-0404.1990.tb01580.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Cermeño J., Echevarría J. M., Villar L. M., Lázaro I., Bootello A., González-Porque P. Soluble class I antigens in serum and CSF of patients with varicella-zoster virus meningitis. J Neurol Neurosurg Psychiatry. 1989 Oct;52(10):1194–1196. doi: 10.1136/jnnp.52.10.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguedas O., Porras O., Fasth A. Juvenile chronic arthritis in Costa Rica. A pilot referral study. Clin Exp Rheumatol. 1995 Jan-Feb;13(1):119–123. [PubMed] [Google Scholar]
- Bresciani A., Pirozzi G., Spera M., Lombardi M. L., Ambrosone L., Migliaresi S., Ferrone S., Manzo C. Increased level of serum HLA class I antigens in patients with systemic lupus in patients with systemic lupus erythematosus. Correlation with disease activity. Tissue Antigens. 1998 Jul;52(1):44–50. doi: 10.1111/j.1399-0039.1998.tb03022.x. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Villar L. M., Leoro G., Alvarez-Cermeño J. C., Roldán E., Gonzalez-Porqué P. Soluble HLA class I antigen secretion by normal lymphocytes: relationship with cell activation and effect of interferon-gamma. Clin Exp Immunol. 1990 Nov;82(2):390–395. doi: 10.1111/j.1365-2249.1990.tb05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouet J. C., Clauvel J. P., Danon F., Klein M., Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med. 1974 Nov;57(5):775–788. doi: 10.1016/0002-9343(74)90852-3. [DOI] [PubMed] [Google Scholar]
- Carbone E., Terrazzano G., Colonna M., Tuosto L., Piccolella E., Franksson L., Palazzolo G., Pérez-Villar J. J., Fontana S., Kärre K. Natural killer clones recognize specific soluble HLA class I molecules. Eur J Immunol. 1996 Mar;26(3):683–689. doi: 10.1002/eji.1830260326. [DOI] [PubMed] [Google Scholar]
- Ferri C., Caracciolo F., Zignego A. L., La Civita L., Monti M., Longombardo G., Lombardini F., Greco F., Capochiani E., Mazzoni A. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br J Haematol. 1994 Oct;88(2):392–394. doi: 10.1111/j.1365-2141.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- Ferri C., Greco F., Longombardo G., Palla P., Moretti A., Marzo E., Fosella P. V., Pasero G., Bombardieri S. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991 Dec;34(12):1606–1610. doi: 10.1002/art.1780341221. [DOI] [PubMed] [Google Scholar]
- Gabrielli A., Manzin A., Candela M., Caniglia M. L., Paolucci S., Danieli M. G., Clementi M. Active hepatitis C virus infection in bone marrow and peripheral blood mononuclear cells from patients with mixed cryoglobulinaemia. Clin Exp Immunol. 1994 Jul;97(1):87–93. doi: 10.1111/j.1365-2249.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorevic P. D., Kassab H. J., Levo Y., Kohn R., Meltzer M., Prose P., Franklin E. C. Mixed cryoglobulinemia: clinical aspects and long-term follow-up of 40 patients. Am J Med. 1980 Aug;69(2):287–308. doi: 10.1016/0002-9343(80)90390-3. [DOI] [PubMed] [Google Scholar]
- Gumber S. C., Chopra S. Hepatitis C: a multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med. 1995 Oct 15;123(8):615–620. doi: 10.7326/0003-4819-123-8-199510150-00008. [DOI] [PubMed] [Google Scholar]
- Hagihara M., Shimura T., Takebe K., Munkhbat B., Hosoi K., Kagawa T., Watanabe N., Matsuzaki S., Yamamoto K., Sato K. Serum concentrations of soluble HLA-class I and CD8 forms in patients with viral hepatic disorders. J Gastroenterol. 1997 Jun;32(3):338–343. doi: 10.1007/BF02934490. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Zavazava N., Steinmann J., Müller-Ruchholtz W. Interaction of papain-digested HLA class I molecules with human alloreactive cytotoxic T lymphocytes (CTL). Clin Exp Immunol. 1993 Jan;91(1):183–188. doi: 10.1111/j.1365-2249.1993.tb03376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao K. J., Scornik J. C., Riley W. J., McQueen C. F. Association between HLA phenotype and HLA concentration in plasma or platelets. Hum Immunol. 1988 Feb;21(2):115–124. doi: 10.1016/0198-8859(88)90086-9. [DOI] [PubMed] [Google Scholar]
- Martin P. Hepatitis C: more than just a liver disease. Gastroenterology. 1993 Jan;104(1):320–323. doi: 10.1016/0016-5085(93)90869-e. [DOI] [PubMed] [Google Scholar]
- McMurray R. W., Elbourne K. Hepatitis C virus infection and autoimmunity. Semin Arthritis Rheum. 1997 Feb;26(4):689–701. doi: 10.1016/s0049-0172(97)80005-4. [DOI] [PubMed] [Google Scholar]
- Meltzer M., Franklin E. C. Cryoglobulinemia--a study of twenty-nine patients. I. IgG and IgM cryoglobulins and factors affecting cryoprecipitability. Am J Med. 1966 Jun;40(6):828–836. doi: 10.1016/0002-9343(66)90199-9. [DOI] [PubMed] [Google Scholar]
- Monteverde A., Ballarè M., Pileri S. Hepatic lymphoid aggregates in chronic hepatitis C and mixed cryoglobulinemia. Springer Semin Immunopathol. 1997;19(1):99–110. doi: 10.1007/BF00945028. [DOI] [PubMed] [Google Scholar]
- Monteverde A., Rivano M. T., Allegra G. C., Monteverde A. I., Zigrossi P., Baglioni P., Gobbi M., Falini B., Bordin G., Pileri S. Essential mixed cryoglobulinemia, type II: a manifestation of a low-grade malignant lymphoma? Clinical-morphological study of 12 cases with special reference to immunohistochemical findings in liver frozen sections. Acta Haematol. 1988;79(1):20–25. doi: 10.1159/000205684. [DOI] [PubMed] [Google Scholar]
- Nocito M., Montalbán C., González-Porque P., Villar L. M. Increased soluble serum HLA class I antigens in patients with lymphoma. Hum Immunol. 1997 Dec;58(2):106–111. doi: 10.1016/s0198-8859(97)00227-9. [DOI] [PubMed] [Google Scholar]
- Onji M., Kikuchi T., Kumon I., Masumoto T., Nadano S., Kajino K., Horiike N., Ohta Y. Intrahepatic lymphocyte subpopulations and HLA class I antigen expression by hepatocytes in chronic hepatitis C. Hepatogastroenterology. 1992 Aug;39(4):340–343. [PubMed] [Google Scholar]
- Pellegrino M. A., Ng A. K., Russo C., Ferrone S. Heterogeneous distribution of the determinants defined by monoclonal antibodies on HLA-A and B antigens bearing molecules. Transplantation. 1982 Jul;34(1):18–23. doi: 10.1097/00007890-198207000-00004. [DOI] [PubMed] [Google Scholar]
- Pickl W. F., Majdic O., Faé I., Reuschel R., Holter W., Knapp W. The soluble pool of beta 2-microglobulin free HLA class I alpha-chains. Qualitative and quantitative characterization. J Immunol. 1993 Sep 1;151(5):2613–2622. [PubMed] [Google Scholar]
- Pozzato G., Mazzaro C., Crovatto M., Modolo M. L., Ceselli S., Mazzi G., Sulfaro S., Franzin F., Tulissi P., Moretti M. Low-grade malignant lymphoma, hepatitis C virus infection, and mixed cryoglobulinemia. Blood. 1994 Nov 1;84(9):3047–3053. [PubMed] [Google Scholar]
- Puppo F., Brenci S., Lanza L., Bosco O., Imro M. A., Scudeletti M., Indiveri F., Ferrone S. Increased level of serum HLA class I antigens in HIV infection. Correlation with disease progression. Hum Immunol. 1994 Aug;40(4):259–266. doi: 10.1016/0198-8859(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Puppo F., Indiveri F., Scudeletti M., Ferrone S. Soluble HLA antigens: new roles and uses. Immunol Today. 1997 Apr;18(4):154–155. doi: 10.1016/s0167-5699(97)84660-9. [DOI] [PubMed] [Google Scholar]
- Puppo F., Picciotto A., Brenci S., Varagona G., Scudeletti M., Ghio M., Balestra V., Celle G., Indiveri F. Behavior of soluble HLA class I antigens in patients with chronic hepatitis C during interferon therapy: an early predictor marker of response? J Clin Immunol. 1995 Jul;15(4):179–184. doi: 10.1007/BF01541087. [DOI] [PubMed] [Google Scholar]
- Puppo F., Scudeletti M., Indiveri F., Ferrone S. Serum HLA class I antigens: markers and modulators of an immune response? Immunol Today. 1995 Mar;16(3):124–127. doi: 10.1016/0167-5699(95)80127-8. [DOI] [PubMed] [Google Scholar]
- Qian C., Camps J., Maluenda M. D., Civeira M. P., Prieto J. Replication of hepatitis C virus in peripheral blood mononuclear cells. Effect of alpha-interferon therapy. J Hepatol. 1992 Nov;16(3):380–383. doi: 10.1016/s0168-8278(05)80674-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K., Koide N., Takenami T., Matsushima H., Takabatake H., Ferrone S., Tsuji T. Soluble HLA class I antigens in sera of patients with chronic hepatitis. Gastroenterol Jpn. 1992 Apr;27(2):206–211. doi: 10.1007/BF02777724. [DOI] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Zavazava N., Hausmann R., Müller-Ruchholtz W. Inhibition of anti-HLA-B7 alloreactive CTL by affinity-purified soluble HLA. Transplantation. 1991 Apr;51(4):838–842. doi: 10.1097/00007890-199104000-00019. [DOI] [PubMed] [Google Scholar]
- Zavazava N., Krönke M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nat Med. 1996 Sep;2(9):1005–1010. doi: 10.1038/nm0996-1005. [DOI] [PubMed] [Google Scholar]
- Zignego A. L., Ferri C., Giannini C., La Civita L., Careccia G., Longombardo G., Bellesi G., Caracciolo F., Thiers V., Gentilini P. Hepatitis C virus infection in mixed cryoglobulinemia and B-cell non-Hodgkin's lymphoma: evidence for a pathogenetic role. Arch Virol. 1997;142(3):545–555. doi: 10.1007/s007050050100. [DOI] [PubMed] [Google Scholar]