Abstract
The mechanism of type II DNA topoisomerases involves the formation of an enzyme-operated gate in one double-stranded DNA segment and the passage of another segment through this gate. DNA gyrase is the only type II topoisomerase able to introduce negative supercoils into DNA, a feature that requires the enzyme to dictate the directionality of strand passage. Although it is known that this is a consequence of the characteristic wrapping of DNA by gyrase, the detailed mechanism by which the transported DNA segment is captured and directed through the DNA gate is largely unknown. We have addressed this mechanism by probing the topology of the bound DNA segment at distinct steps of the catalytic cycle. We propose a model in which gyrase captures a contiguous DNA segment with high probability, irrespective of the superhelical density of the DNA substrate, setting up an equilibrium of the transported segment across the DNA gate. The overall efficiency of strand passage is determined by the position of this equilibrium, which depends on the superhelical density of the DNA substrate. This mechanism is concerted, in that capture of the transported segment by the ATP-operated clamp induces opening of the DNA gate, which in turn stimulates ATP hydrolysis.
Keywords: supercoiling, topoisomerase, energy coupling
The topological state of DNA plays an important role in its biological activity and is maintained by a group of enzymes called DNA topoisomerases (1, 2). These enzymes perform the formidable task of passing one DNA segment through another. This feat is achieved by the cleavage of DNA and the formation of a transient DNA gate through which a segment of the same or another DNA molecule is passed. Mechanistic differences in this reaction distinguish topoisomerases into two types. Type I enzymes introduce a single-stranded break in DNA and facilitate the passage of a second strand through this gate. Type II topoisomerases create a double-stranded break and allow the passage of a double-stranded DNA segment.
Type II topoisomerases are able to catalyze a number of reactions, including the negative supercoiling of DNA, the relaxation of negative and positive supercoils, the catenation and decatenation of DNA circles, and the knotting and unknotting of DNA (3). These reactions generally require the hydrolysis of ATP. Most type II enzymes [e.g., prokaryotic topoisomerase (topo) IV and eukaryotic topo II] favor DNA relaxation and decatenation reactions. DNA gyrase is the only topoisomerase able to introduce negative supercoils into DNA in an ATP-dependent manner (3, 4). All type II enzymes share a degree of sequence similarity but tend to differ at their C termini (5). DNA gyrase is composed of two subunits, DNA gyrase A protein (GyrA) (97 kDa in Escherichia coli) and DNA gyrase B protein (GyrB) (90 kDa in E. coli), the active form being an A2B2 heterotetramer. Eukaryotic topo IIs are homodimers where the monomer is equivalent to a fusion of the A and B subunits of gyrase, such that the N terminus is homologous to the B subunit, and the C terminus corresponds approximately to the A subunit (6). Topoisomerases are essential to all cells and as such, they are important targets of antibacterial and antineoplastic drugs (7, 8).
Type II topoisomerases are believed to operate as molecular clamps (9, 10). The enzymes bind a DNA segment (the gate or G segment), which is cleaved in both strands with covalent attachment of the phosphate backbone to a pair of tyrosines on the enzyme, forming a transient DNA gate. The N-terminal domains of the enzyme complex (of the GyrB subunits in the case of gyrase) form a protein clamp that dimerizes in the presence of ATP, trapping another DNA segment (the transported or T segment), which is passed through the DNA gate. When the two segments (G and T) are part of different DNA molecules, this mechanism results in catenation/decatenation. When the two segments are part of the same molecule, strand passage generally results in DNA relaxation. However, in the presence of ATP, DNA gyrase can introduce negative supercoils into DNA, a reaction that requires the enzyme to dictate the orientation of the T segment with respect to the DNA gate. Gyrase differs from the other type II enzymes in its interaction with DNA. It wraps ≈130 bp of DNA in a positive superhelical sense, whereas the other enzymes interact with 35 bp or less without significant distortion of the DNA conformation (11–13). The supercoiling ability of gyrase appears to derive from this unique right-handed wrapping of the DNA, since deletion of the C-terminal DNA-binding domain of GyrA, which is responsible for the wrap, abolishes supercoiling in favor of relaxation and decatenation (14), reactions more typical of the other type II enzymes. However, the mechanism by which this interaction directs the T segment to the ATP-operated clamp and the details of the strand-passage reaction are still unclear.
Binding of the nonhydrolysable ATP analog adenosine 5′-[β,γ-imido]triphosphate (ADPNP) traps the clamp in the dimerized form and can support a single strand-passage step (15). The efficiency of this reaction was found to depend on the topological state of the DNA; with positively supercoiled DNA, the efficiency of strand passage is almost 100%, decreasing to ≈25% with relaxed, and to minimal levels with negatively supercoiled DNA (16). It has been proposed that the efficiency of strand passage depends on the probability with which the T segment is captured (16). Thus, positively supercoiled molecules, which readily form the positively wrapped conformation, are better substrates than relaxed or negatively supercoiled ones. Moreover, it is proposed that once the T segment is trapped, it is passed through the DNA gate with high probability, whereas nucleotide hydrolysis is required for enzyme turnover (16). However, a number of observations appear to contradict this model. Firstly, it has been suggested that the presence of a DNA segment in the ATP-operated clamp stimulates the rate of ATP hydrolysis (17, 18). Therefore, if capture of the T segment depended on the topological state of the DNA, so would the rate of ATP hydrolysis. However, the rate of hydrolysis is the same in the presence of excess linear, relaxed, or negatively supercoiled DNA (14, 16, 19). Secondly, the thiophosphate ATP analog ATPβS(Rp) cannot support efficient supercoiling, although it is hydrolyzed as well as ATP, suggesting that the efficiency of strand passage is determined by an event subsequent to T-segment capture (20). Thirdly, it is not obvious how a model in which the limit of supercoiling depends on the probability of capture of a T segment explains the experimental evidence suggesting that the limit is determined by the thermodynamics of ATP hydrolysis (21–23).
To address the mechanism of strand passage, we used DNA relaxation by eukaryotic topo I as a topological probe. Topo I is an ATP-independent topoisomerase that relaxes DNA in the thermodynamically favorable direction (24). Treatment of a protein-bound closed-circular DNA molecule with topo I relaxes the protein-free DNA but not the part stabilized by the protein. Therefore, if protein binding induces changes in the twist and writhe of the DNA, analysis of the topo I-treated DNA after removal of the protein can provide useful information on the conformation of the protein–DNA complex.
MATERIALS AND METHODS
Enzymes and DNA.
E. coli GyrA, GyrASer122, GyrA64, GyrA64Phe122, GyrA64Ser122 (gifts of S. E. Critchlow), and GyrB (gift of A. J. Howells) were prepared as described previously (25). Chicken blood topo I (gift of A. J. Howells) was prepared as described by Trask and Muller (26). Positively supercoiled DNA was prepared by incubating relaxed pBR322 (gift of A. J. Howells) with gyrase and topo I under the conditions described for the topo I relaxation assays.
Enzyme Assays.
ATPase assays were performed as described previously (27). Topo I relaxation assays (30 μL) contained 0.8 μg of the appropriate topological form of pBR322 and the indicated amounts of enzyme in 35 mM Tris⋅HCl (pH 7.5)/24 mM KCl/4 mM MgCl2/1.8 mM spermidine/0.36 mg/ml BSA/9 μg/ml tRNA. Where indicated, ADPNP was added to 1.4 mM, and the reactions were incubated for 30 min at 25°C. Samples were then treated with topo I for 30 min at 37°C. The final concentration of topo I was a six-fold excess of the concentration required to fully relax the same amount of negatively supercoiled pBR322 (σ = −0.06) under the above conditions. Reactions were stopped by the addition of SDS to 0.2% and treated with 0.1 mg/ml proteinase K for 30 min at 37°C. The DNA was extracted with chloroform/isoamyl alcohol, 25:1 (vol/vol), and analyzed by 1% agarose gel electrophoresis in 90 mM Tris/90 mM acetic acid/1 mM EDTA, in the presence of 0–3 μg/ml chloroquine as appropriate (28). ADPNP-driven single-strand passage experiments were performed in a similar manner to the topo I relaxation assays except for the omission of the topo I relaxation step. Experiments with A642Phe122B2 were all performed under ATP-dependent relaxation conditions (14). Linking number changes were measured by determining the center of the topoisomer distribution after capture of the gel image by camera and analysis of the image by using the program imagequant (Molecular Dynamics).
RESULTS AND DISCUSSION
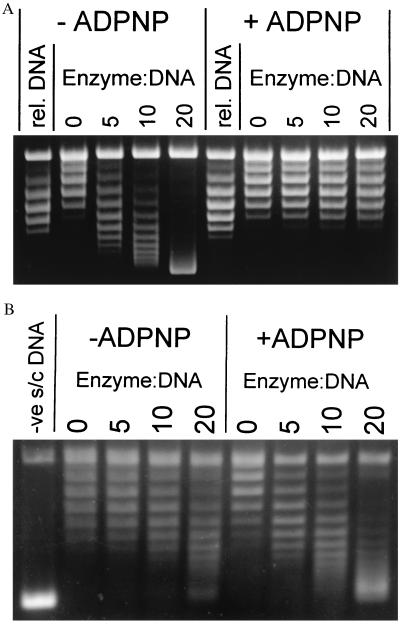
To test whether the efficiency of T-segment capture depends on the topological state of the DNA, gyrase–DNA complexes formed at different superhelical densities (specific linking differences; σ) were incubated with ADPNP and then probed with topo I to assess the conformation of the bound DNA segment. As a control, gyrase was also incubated with relaxed, negatively and positively supercoiled pBR322 DNA (σ = 0, −0.06 and +0.03 respectively) and treated with topo I in the absence of nucleotide. In this case, all three DNAs yielded the expected linking difference of ≈+0.8 per enzyme (ΔLk/Enz. = +0.8), characteristic of the wrapping of DNA around gyrase (4) (Fig. 1A and Table 1). However, after formation of the enzyme–DNA–ADPNP complex and treatment with topo I, the DNA was found to be almost fully relaxed (ΔLk/Enz. < +0.05), independent of the initial topological state of the substrate (Fig. 1A and Table 1). Similar experiments, in which religation of singly nicked DNA with E. coli DNA ligase was used instead of topo I relaxation, corroborate these findings (M. H. O’Dea and M. Gellert, personal communication). When the ability of the enzyme to perform ADPNP-induced supercoiling was investigated, each gyrase tetramer introduced 0.55 negative supercoils into relaxed DNA, in good agreement with previous observations (16).
Figure 1.
Topo I relaxation reaction of gyrase–DNA complexes. (A) Typical assay showing the relaxation by topo I of the gyrase-DNA and gyrase–DNA–ADPNP complexes formed on relaxed DNA. The starting material is shown in the track labeled “rel. DNA.” Numbers above the tracks indicate enzyme/DNA ratios; the DNA concentration was 9.4 nM. (Small differences in superhelical density between the starting material and the product of topo I relaxation without gyrase are caused by the different conditions used in the preparation of relaxed DNA.) (B) Trapping of the T segment in the ATP-operated clamp is demonstrated by a similar experiment using A642Phe122B2 and negatively supercoiled DNA. The starting DNA (σ = −0.06) is shown in the track labeled “-ve s/c DNA.” Ciprofloxacin (200 μM) was present in this experiment to stabilize the weak enzyme–DNA complex (14); however, similar results were obtained in the absence of the quinolone. A642Ser122B2 also produced similar results with or without the drug.
Table 1.
Summary of results of topo I topological assays
| Nucleotide | DNA topological form | ΔLk/Enz.
|
||
|---|---|---|---|---|
| A2B2 | A2Ser122B2 | A642Phe122B2 | ||
| −ADPNP | −ve s/c (σ = −0.06) | +0.80 ± 0.1 | +0.80 ± 0.2 | −0.20 ± 0.05 |
| Relaxed | +0.70 ± 0.1 | +0.60 ± 0.1 | — | |
| +ve s/c (σ = +0.03) | +0.80 ± 0.1 | +0.60 ± 0.1 | — | |
| +ADPNP | −ve s/c (σ = −0.06) | +0.05 ± 0.05 | +0.20 ± 0.05 | −0.45 ± 0.05 |
| Relaxed | +0.00 ± 0.05 | +0.15 ± 0.05 | — | |
| +ve s/c (σ = +0.03) | +0.05 ± 0.05 | +0.15 ± 0.05 | — | |
Results are reported as the average of a number of different experiments; errors express the range of experimental values.
To study the relationship between strand passage and the linking number changes observed, we used an active-site mutant of gyrase (GyrASer122) that cannot cleave DNA and thus cannot perform strand passage (29). With all three topological forms of the DNA substrate, A2Ser122B2 was found to induce a ΔLk/Enz. of ≈+0.7 per enzyme in the absence of ADPNP, i.e., very similar to wild type. When ADPNP was bound, ΔLk/Enz. was ≈+0.2 in all cases (Table 1).
These results seem to contradict the model for the mechanism of strand passage that predicts that, depending on the topological form of the DNA, a different proportion of the enzyme will trap the T segment in the presence of ADPNP (the “probability of capture” model) (16). To reconcile the results obtained with the wild-type enzyme with this model, we would have to assume that the complexes that have trapped and transported the T segment have a similar conformation to those where clamp closure failed to trap a segment and that, in each case, the overall wrapping of the DNA is almost zero. A model that would be consistent with this is one in which after strand passage, or after failure to trap a T segment, the wrapped DNA is released from the enzyme. However, this idea seems inconsistent with a number of experiments that support the retention of the wrap in the presence of ADPNP and indeed show increased protection of the bound DNA from nucleases and other agents. These include DNase I footprinting (30–33), exonuclease protection (31, 33), and hydroxyl radical footprinting (12). In the case of the latter two methods, the data are consistent with further wrapping of the DNA onto the enzyme core. Given these results, if the probability of capture model is correct, we must assume that the DNA remains bound on the enzyme but in each case (i.e., where the T segment is trapped and transported and where it is not trapped), the overall ΔLk of the bound segment is approximately zero.
However, this interpretation cannot account for the results obtained with A2Ser122B2 (which cannot cleave DNA), provided the ability of this mutant to trap the T segment within the ATP-operated clamp is unaffected. The explanation for this conclusion is as follows. The topology of the crossover formed by the trapped T segment and the segment that contains the DNA gate must be positive in order for gyrase to perform supercoiling. If T-segment capture did depend on the topological state of the DNA, topo I treatment of the A2Ser122B2–ADPNP complex formed on positively supercoiled DNA would be expected to produce a positive linking difference, because almost all the T segments would be trapped and remain in the ATP-operated clamp. In contrast, in the complex formed on negatively supercoiled DNA, where almost no DNA is trapped, ΔLk/Enz. would be expected to be approximately zero, consistent with the wild-type enzyme results in Table 1. In fact, the ΔLk/Enz. value of the A2Ser122B2–ADPNP complex is positive (≈+0.2) in all cases (Table 1).
We thus suggest that, in the presence of ADPNP, A2Ser122B2 does capture a T segment. However, this cannot be deduced unambiguously from the topo I data for this complex. Support for the idea that the T segment can be trapped inside the dimerized clamp when the DNA gate is not allowed to open comes from experiments with the active-site mutant of the C-terminal truncated version of gyrase (A642B2). This enzyme does not wrap DNA (14), thus making it easier in a topo I experiment to identify whether a DNA crossover has been trapped. Binding of A642Phe122B2 to negatively supercoiled DNA stabilized ≈0.20 negative crossovers per enzyme (Fig. 1B). However, when ADPNP was bound to the mutant complexes, the number of negative crossovers trapped increased to ≈0.45 per enzyme, consistent with T-segment trapping (Fig. 1B; A642Ser122B2 produced similar results). Alternatively, it is possible that the complex stabilized by A642Phe122B2 consists of a G segment and another segment of DNA that is not the T segment, as has been suggested in the case of topo II (39), and the addition of ADPNP leads to further stabilization of this alternative complex. However, there is currently no evidence for the existence of such complexes with gyrase and its derivatives. Another possible explanation for the ΔLk change observed in the presence of the nucleotide is that it is caused by a conformational change in the A642Phe122B2 complex, which is characteristic of the inability of this enzyme to cleave DNA. However as A642Phe122B2 binds only 20–25 bp of DNA (14), a change in the linking number of ≈−0.45 appears rather large, perhaps making this an unlikely explanation.
Thus, it seems that our results are hard to reconcile with the probability of capture model (16). As a simpler explanation of our observations, we propose that the T segment is captured with high probability on nucleotide binding, irrespective of the topology of the DNA substrate. In the absence of ADPNP, DNA gyrase stabilizes a positive writhe of ≈0.8 (Table 1), consistent with previous observations (4). In the presence of ADPNP, the T segment is trapped and is not irreversibly released from the enzyme after it has passed the DNA gate; instead, it is able to equilibrate within the enzyme between the two sides of the DNA gate. The position of this equilibrium will depend on the topology of the DNA substrate. Thus, with positively supercoiled substrates, the T segment would equilibrate predominantly beyond the gate, and stopping the reaction at that point would reveal efficient strand passage. With negatively supercoiled substrates, however, the T segment would be predominantly before the DNA gate, within the ATP-operated clamp, and very little strand passage would be observed. Topo I treatment of gyrase–ADPNP complexes formed with DNA of different superhelical densities would relax the part of the DNA that is not constrained by the enzyme. Thus, the T segment would reach an equilibrium within the enzyme that is independent of the topological state of the starting material, consistent with the independence of ΔLk on substrate in our experiments. The very low value of ΔLk arises from the sum of a positive contribution from those complexes where the T segment is before the gate and a negative one from those where it is beyond the gate. The simplest hypothesis for the role of ATP hydrolysis would be that it is required for release of the T segment from the enzyme. However, this idea is in conflict with the apparent situation in yeast topo II, where decatenation experiments have suggested that the T segment is released from the enzyme on ADPNP binding (34). This discrepancy could be attributed to intrinsic differences between the two enzymes. Alternatively, it is possible that the T segment is free to equilibrate not only across the DNA gate within the enzyme, but also across the interface between the A subunits (the C gate). In the circumstances of an energetically favorable topo II decatenation experiment, this would result in the appearance of free product, as seen by Roca and Wang (34). However, in the case of gyrase, where the T segment is constrained by being close to or part of the wrapped region (see below), the equilibrium position after the topo I relaxation experiment described here could be with the DNA mostly within the complex.
At the structural level, the information on gyrase is partial. The structure of the 59-kDa N-terminal domain of GyrA and the 43-kDa N-terminal domain of GyrB have been solved independently (35, 36), and the structure of a 92-kDa fragment of yeast topo II homologous to the N-terminal domain of GyrA and the 47-kDa C-terminal domain of GyrB has also been solved (10). Taken together with electron microscopy studies on yeast topo II (37), the spatial arrangement of the gyrase subunits can be proposed to be similar to that shown in Fig. 2. In this model, the unknown structure of the 47-kDa domain of GyrB is replaced by that of the homologous domain of yeast topo II. The structure of the 33-kDa C-terminal domain of GyrA is also unknown. This domain is the major component of the DNA-binding surface of gyrase and contributes ≈100 bp of positive wrap, whereas its deletion abolishes the ability of gyrase to dictate the direction of strand passage (14). We suggest that the 33-kDa domain is located such that it directs the T segment to the ATP-operated clamp. Moreover, to achieve this high efficiency of trapping, the T segment must be very close to or part of the wrapped segment. This is consistent with the observation that gyrase can carry out strand-passage reactions on very small DNA circles (22) and is supported by hydroxyl-radical footprinting experiments that revealed an extension in the protection of one of the two DNA arms in the presence of ADPNP (12). We propose that the 33-kDa domain extends from the DNA gate toward the entrance to the clamp as shown in Fig. 2a. In the nucleotide-free complex, the DNA is wrapped in a positive configuration (Fig. 2a); the proposed DNA-binding domain of GyrB (18, 36) could contribute to the wrapping of the bound segment.
Figure 2.
A model for the organization of the gyrase tetramer and the mechanism of strand passage. (a) The nucleotide-free conformation is shown, consisting of the 59-kDa domain of GyrA [green-blue (35)], the 43-kDa domain of GyrB [purple (36)] and the B′ subdomain of yeast topo II [red (10)], their relative positions suggested by electron microscopy observations (37). The structure of the 33-kDa domain is unknown; a representation is given here (yellow). The conformation of the bound DNA is shown here to be symmetrical. However, hydroxyl radical experiments show a preference for the protection of one of the DNA arms (12). Such protection must be the result of differences in the flexibility of the two arms and suggests that DNA structure plays a role in determining which of the two arms is captured by the ATP-operated clamp. (b) The conformation of the enzyme–DNA complex when the T segment is before the DNA gate. (c) The enzyme–DNA complex when the T segment is beyond the DNA gate. The conformational changes that are depicted to take place on nucleotide binding are discussed in the text. In the gate-open conformation, the structure of the 59-kDa domain has been substituted with that of the A′ subdomain of yeast topo II (10). In the nucleotide-bound complex (complexes b and c), the DNA gate is shown in the open state merely to illustrate the opening of the gate to allow strand passage. At equilibrium, this gate may be predominantly in the closed state, as suggested by the cleavage experiments in Fig. 3.
To facilitate passage of the T segment through the DNA gate, large movements of enzyme and DNA must accompany the binding of the nucleotide (Fig. 2). These movements are reflected in the changes in DNA conformation observed in these experiments. Moreover, comparison of the crystal structures of the 59-kDa N-terminal domain of GyrA and the 92-kDa fragment of yeast topo II suggests that there are large conformational changes on gate opening. In the case of A2Ser122B2, the overall wrapping drops to +0.2 turns per enzyme after the binding of the nucleotide. Because in this complex we propose that a positive node is always trapped, a negative distortion may be required elsewhere in the bound DNA in order for the overall ΔLk/Enz. to be +0.2. To account for this, in the model shown in Fig. 2b we suggest that, when the T segment is before the DNA gate, the conformation of the DNA arm that has not been trapped may be that of a negative superhelix.
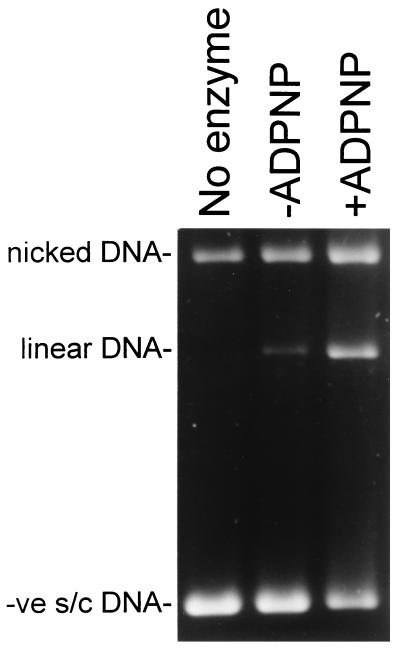
The above model suggests that the presence of the T segment in the ATP-operated clamp might affect the DNA cleavage-religation equilibrium established between gyrase and the DNA segment that forms the DNA gate. To test this hypothesis, the dependence of ADPNP-induced cleavage on DNA topology was investigated. In the absence of nucleotide, the DNA cleavage-religation equilibrium is predominantly in the ligated form, because interrupting the enzyme–DNA complex with a protein denaturant reveals only minimal amounts of cleavage. However, binding of ADPNP (Fig. 3) or ATP (data not shown) shifts this equilibrium, increasing the extent of cleavage [see also Critchlow et al. (38)]. With negatively supercoiled DNA, where we propose that the T segment is predominantly in the ATP-operated clamp, ADPNP-induced cleavage was ≈8 times more efficient than in the absence of the nucleotide, whereas with relaxed DNA the efficiency of cleavage was increased by a factor of ≈3 (Table 2). However, with positively supercoiled DNA, where we propose that the T segment is predominantly beyond the DNA gate, the extent of ADPNP-induced cleavage was similar to that observed in the absence of the nucleotide (Table 2). These results were corroborated in another way. In this case, the ability of gyrase to perform ADPNP-induced cleavage of a stoichiometric amount of radiolabeled 80-bp fragment in the presence of excess unlabeled 40-bp fragment was studied. Because of its higher affinity for gyrase, the 80-bp fragment is expected to behave as a G segment, whereas the 40-mer is expected to play the role of the T segment. Gyrase supported similar very low levels of cleavage of the 80-bp fragment in the absence or presence of ADPNP. However, the presence of the 40-bp fragment resulted in significant increase in the levels of ADPNP-induced cleavage of the 80 mer (data not shown). This result provides further support for the idea that the presence of the T segment inside the ATP-operated clamp shifts the equilibrium toward DNA cleavage.
Figure 3.
The presence of the T segment in the ATP-operated clamp promotes DNA cleavage. Negatively supercoiled DNA (10 nM; σ = −0.06) was incubated with 200 nM gyrase and 1.4 mM ADPNP, where indicated. The samples were treated with 0.2% SDS and 0.1 mg/ml proteinase K for 30 min at 37°C to reveal DNA cleavage, and the products were analyzed by agarose gel electrophoresis.
Table 2.
Dependence of ADPNP-induced cleavage on DNA topology
| DNA topological form | Cleavage efficiency %
|
Ratio | |
|---|---|---|---|
| −ADPNP | +ADPNP | ||
| −ve s/c | 0.16 ± 0.07 | 1.22 ± 0.20 | 7.6 ± 2.0 |
| Relaxed | 0.19 ± 0.04 | 0.58 ± 0.10 | 3.1 ± 0.5 |
| +ve s/c | 0.49 ± 0.20 | 0.68 ± 0.02 | 1.4 ± 0.3 |
Results are reported as the average of a number of different experiments; errors express the range of experimental values.
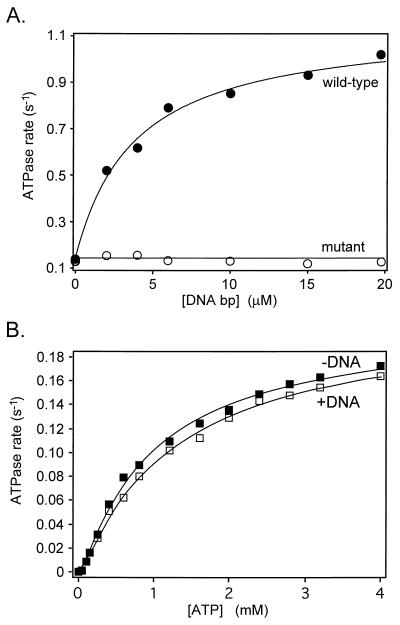
It has been proposed that the presence of a DNA segment in the ATP-operated clamp stimulates the rate of ATP hydrolysis (17, 18). Therefore, T-segment capture appears to be related to both DNA cleavage and ATPase stimulation. We investigated the relationship between DNA cleavage and ATP hydrolysis by studying the ATPase activity of the active-site mutant A2Ser122B2. In this mutant, DNA binding did not stimulate the rate of ATP hydrolysis (Fig. 4A). However, its inability to cleave DNA did not affect the intrinsic ATPase reaction of the mutant, since the kinetics of ATP hydrolysis in the presence or absence of DNA are identical (Fig. 4B), i.e., cleavage is not required for the intrinsic ATPase of the complex but may be necessary for the DNA-stimulated reaction. This result provides further support for the proposition that the active site mutant can capture a T segment. If a T segment cannot be trapped, then its presence in the clamp before ATP binding (approximately 25% of the time, according to the probability of capture model) would be expected to inhibit the intrinsic ATPase of the mutant. The fact that this inhibition is not observed (Fig. 4B) is consistent with the idea that the mutant does trap a T segment. Assuming this to be the case, it appears that DNA binding in the ATP-operated clamp is not sufficient on its own to induce the ATPase of gyrase, and T segment-induced DNA cleavage is required for ATPase stimulation. Further support for this notion comes from recent findings in yeast topo II (40, 41).
Figure 4.
ATPase activity of wild-type gyrase and an active-site mutant. (A) DNA binding to the active-site mutant (A2Ser122B2) is unable to stimulate the rate of ATP hydrolysis. Reactions contained 20 nM gyrase, 2 mM ATP, and the indicated amounts of linear pBR322 DNA; rates were measured at 25°C. (B) The kinetics of ATP hydrolysis by A2Ser122B2. Samples contained 20 nM enzyme and 5 nM linear pBR322 DNA, where indicated. Reactions were carried out at 25°C. The data were fitted to the model described by Kampranis and Maxwell (42).
Taken together, these results suggest that a concerted mechanism operates in DNA gyrase, in which trapping of a T segment promotes cleavage of the DNA at the gate and the setting up of an on-enzyme equilibrium. T segment-induced cleavage triggers ATP hydrolysis, which completes the catalytic turnover of the enzyme.
CONCLUSIONS
Previous work had supported a model for gyrase-catalyzed supercoiling in which the probability of T-segment capture by the ATP-operated clamp depended on the topological state of the DNA (16). Positively supercoiled DNA substrates led to high efficiency of capture, whereas negatively supercoiled DNA was captured with low efficiency. Experiments using topo I as a probe for the topology of gyrase–DNA complexes now suggest that a T segment is captured with high efficiency irrespective of the topology of the DNA substrate. We propose that this capture is enabled by the wrapped conformation of the gyrase–DNA complex, whereby the 33-kDa C-terminal domains of GyrA deliver the T segment to the ATP-operated clamp (Fig. 2). Indeed, it is feasible that the N-terminal ATP-binding domain of GyrB forms part of the wrap surface. The probability of subsequent strand passage depends on an on-enzyme equilibrium, the position of which is determined by DNA topology; positively supercoiled substrates undergo strand passage with a high probability [as manifested by their high coupling ratios in ADPNP strand-passage reactions (16)], and negatively supercoiled DNAs are poor substrates for the strand-passage reaction. T-segment capture shifts the DNA cleavage-religation equilibrium toward the cleaved form, an event that acts as a trigger for ATP hydrolysis and enables the free energy of ATP hydrolysis and product release to be coupled to enzyme turnover.
Acknowledgments
We thank A. J. Howells and S. E. Critchlow for gifts of enzyme and DNA, and M. H. O’Dea and N. L. Williams for comments on the manuscript. This work was supported by Biotechnology and Biological Sciences Research Council, Glaxo Wellcome, the A. S. Onassis Public Benefit Foundation (S.C.K.), and the Wellcome Trust (A.D.B.). A.M. was a Lister Institute Jenner Fellow.
ABBREVIATIONS
- ADPNP
adenosine 5′-[β,γ-imido]triphosphate
- GyrA
DNA gyrase A protein
- GyrB
DNA gyrase B protein
- topo
topoisomerase
References
- 1.Bates A D, Maxwell A. DNA Topology. Oxford: IRL; 1993. [Google Scholar]
- 2.Wang J C. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 3.Wang J C. Quart Rev Biophys. 1998;31:107–144. doi: 10.1017/s0033583598003424. [DOI] [PubMed] [Google Scholar]
- 4.Reece R J, Maxwell A. CRC Crit Rev Biochem Mol Biol. 1991;26:335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- 5.Caron P R, Wang J C. Adv Pharmacol. 1994;29B:271–297. doi: 10.1016/s1054-3589(08)61143-6. [DOI] [PubMed] [Google Scholar]
- 6.Lynn R, Giaever G, Swanberg S, Wang J C. Science. 1986;233:647–648. doi: 10.1126/science.3014661. [DOI] [PubMed] [Google Scholar]
- 7.Maxwell A. Trends Microbiol. 1997;5:102–109. doi: 10.1016/S0966-842X(96)10085-8. [DOI] [PubMed] [Google Scholar]
- 8.Froelich-Ammon S J, Osheroff N. J Biol Chem. 1995;270:21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- 9.Roca J, Berger J M, Harrison S C, Wang J C. Proc Natl Acad Sci USA. 1996;93:4057–4062. doi: 10.1073/pnas.93.9.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger J M, Gamblin S J, Harrison S C, Wang J C. Nature (London) 1996;379:225–232. doi: 10.1038/379225a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee M P, Sander M, Hsieh T. J Biol Chem. 1989;264:21779–21787. [PubMed] [Google Scholar]
- 12.Orphanides G, Maxwell A. Nucleic Acids Res. 1994;22:1567–1575. doi: 10.1093/nar/22.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng H, Marians K J. J Biol Chem. 1995;270:25286–25290. doi: 10.1074/jbc.270.42.25286. [DOI] [PubMed] [Google Scholar]
- 14.Kampranis S C, Maxwell A. Proc Natl Acad Sci USA. 1996;93:14416–14421. doi: 10.1073/pnas.93.25.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugino A, Higgins N P, Brown P O, Peebles C L, Cozzarelli N R. Proc Natl Acad Sci USA. 1978;75:4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bates A D, O’Dea M H, Gellert M. Biochemistry. 1996;35:1408–1416. doi: 10.1021/bi952433y. [DOI] [PubMed] [Google Scholar]
- 17.Maxwell A, Gellert M. J Biol Chem. 1984;259:14472–14480. [PubMed] [Google Scholar]
- 18.Tingey A P, Maxwell A. Nucleic Acids Res. 1996;24:4868–4873. doi: 10.1093/nar/24.24.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugino A, Cozzarelli N R. J Biol Chem. 1980;255:6299–6306. [PubMed] [Google Scholar]
- 20.Cullis P M, Maxwell A, Weiner D P. Biochemistry. 1997;36:6059–6068. doi: 10.1021/bi962725e. [DOI] [PubMed] [Google Scholar]
- 21.Westerhoff H V, O’Dea M H, Maxwell A, Gellert M. Cell Biophys. 1988;12:157–181. doi: 10.1007/BF02918357. [DOI] [PubMed] [Google Scholar]
- 22.Bates A D, Maxwell A. EMBO J. 1989;8:1861–1866. doi: 10.1002/j.1460-2075.1989.tb03582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullis P M, Maxwell A, Weiner D P. Biochemistry. 1992;31:9642–9646. doi: 10.1021/bi00155a017. [DOI] [PubMed] [Google Scholar]
- 24.Gupta M, Fujimori A, Pommier Y. Biochim Biophys Acta. 1995;1262:1–14. doi: 10.1016/0167-4781(95)00029-g. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell A, Howells A J. In: Protocols for DNA Topoisomerases I. DNA Topology and Enzyme Purification. Bjornsti M-A, Osheroff N, editors. Towata, NJ: Humana; 1999. pp. 135–144. [Google Scholar]
- 26.Trask D K, Muller M T. Nucleic Acids Res. 1983;11:2779–2800. doi: 10.1093/nar/11.9.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali J A, Jackson A P, Howells A J, Maxwell A. Biochemistry. 1993;32:2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- 28.Shure M, Pulleyblank D, Vinograd J. Nucleic Acids Res. 1977;4:1183–1205. doi: 10.1093/nar/4.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Critchlow S E, Maxwell A. Biochemistry. 1996;35:7387–7393. doi: 10.1021/bi9603175. [DOI] [PubMed] [Google Scholar]
- 30.Fisher L M, Mizuuchi K, O’Dea M H, Ohmori H, Gellert M. Proc Natl Acad Sci USA. 1981;78:4165–4169. doi: 10.1073/pnas.78.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison A, Cozzarelli N R. Proc Natl Acad Sci USA. 1981;78:1416–1420. doi: 10.1073/pnas.78.3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirkegaard K, Wang J C. Cell. 1981;23:721–729. doi: 10.1016/0092-8674(81)90435-9. [DOI] [PubMed] [Google Scholar]
- 33.Rau D C, Gellert M, Thoma F, Maxwell A. J Mol Biol. 1987;193:555–569. doi: 10.1016/0022-2836(87)90266-x. [DOI] [PubMed] [Google Scholar]
- 34.Roca J, Wang J C. Cell. 1994;77:609–616. doi: 10.1016/0092-8674(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 35.Morais Cabral J H, Jackson A P, Smith C V, Shikotra N, Maxwell A, Liddington R C. Nature (London) 1997;388:903–906. doi: 10.1038/42294. [DOI] [PubMed] [Google Scholar]
- 36.Wigley D B, Davies G J, Dodson E J, Maxwell A, Dodson G. Nature (London) 1991;351:624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- 37.Schultz P, Olland S, Oudet P, Hancock R. Proc Natl Acad Sci USA. 1996;93:5936–5940. doi: 10.1073/pnas.93.12.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Critchlow S E, O’Dea M H, Howells A J, Couturier M, Gellert M, Maxwell A. J Mol Biol. 1997;273:826–839. doi: 10.1006/jmbi.1997.1357. [DOI] [PubMed] [Google Scholar]
- 39.Roca J, Berger J M, Wang J C. J Biol Chem. 1993;268:14250–14255. [PubMed] [Google Scholar]
- 40.Harkins T T, Lindsley J E. Biochemistry. 1998;37:7292–7298. doi: 10.1021/bi9729099. [DOI] [PubMed] [Google Scholar]
- 41.Harkins T T, Lewis T J, Lindsley J E. Biochemistry. 1998;37:7299–7312. doi: 10.1021/bi9729108. [DOI] [PubMed] [Google Scholar]
- 42.Kampranis S C, Maxwell A. J Biol Chem. 1998;273:22615–22626. doi: 10.1074/jbc.273.35.22615. [DOI] [PubMed] [Google Scholar]