Abstract
OBJECTIVE—To determine the effect of the thrombin inhibitor, hirudin, on the pathogenesis of murine antigen induced arthritis (AIA). METHODS—AIA was induced by intra-articular injection of methylated bovine serum albumin in the knee joints of previously immunised mice. Hirudin (injected subcutaneously 3 × 200 µg/mouse/day) was given over 13 days, starting three days before arthritis onset, and its anticoagulant effect monitored by clotting times. Arthritis severity was evaluated by technetium-99m (99mTc) uptake in the knee joints and by histological scoring. In addition, intra-articular fibrin deposition was examined by immunohistochemistry, and synovial cytokine mRNA expression measured by RNase protection. RESULTS—Joint inflammation, measured by 99mTc uptake, was significantly reduced in hirudin treated mice at days 7 and 10 after arthritis onset. Histologically, synovial thickness was markedly decreased in hirudin treated mice compared with untreated ones. By contrast, no difference in articular cartilage proteoglycan content was found between both groups. Intra-articular fibrin deposition and synovial interleukin 1β mRNA levels, were slightly reduced (~20%) in arthritic joints from hirudin treated mice compared with untreated ones at day 10 of AIA. CONCLUSION—Hirudin reduces joint inflammation associated with AIA by fibrin-dependent and independent mechanisms.
Full Text
The Full Text of this article is available as a PDF (940.4 KB).
Figure 1 .
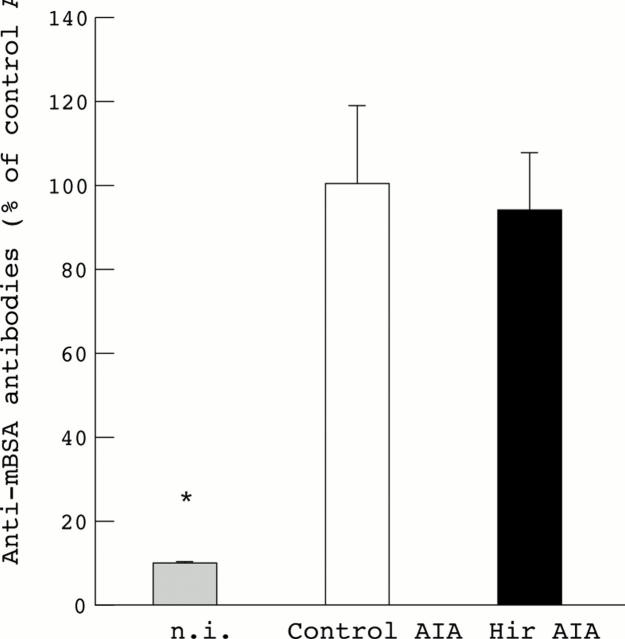
Anti-methylated bovine serum albumin (anti-mBSA) antibody levels in control and hirudin treated mice with antigen induced arthritis (AIA). The results were expressed as a percentage of the anti-mBSA antibody content in the plasma of untreated arthritic mice. There was no significant difference between immunisations in untreated (n=11) and treated (n=12) arthritic mice. Non-immunised (n.i.), non-arthritic mice (n=5), were used as negative controls. Results are expressed as means (SEM). Statistical significance was tested by Student's t test. *p<0.05 was considered significant.
Figure 2 .
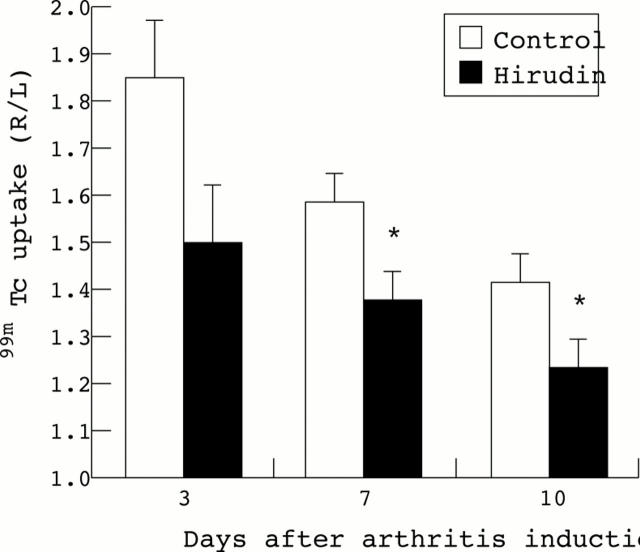
Time course of knee joint inflammation in hirudin treated mice with antigen induced arthritis. Joint inflammation was measured by external gamma counting of 99mTc uptake on days 3, 7, and 10 after antigen challenge into the right knee. Results are expressed as the ratio of 99mTc uptake in the right (R) arthritic knee joint over the left (L) non-inflamed contralateral knee joint, a value higher than 1.1 indicating joint inflammation. For each time point the mean (SEM) of the ratios is shown. Control (n=11), hirudin day 3 (n=12), days 7 and 10 (n=11). Statistical significance was tested by Student's t test. The p values were 0.108 (day 3); 0.028 (day 7); 0.035 (day 10). *p<0.05 was considered significant.
Figure 3 .
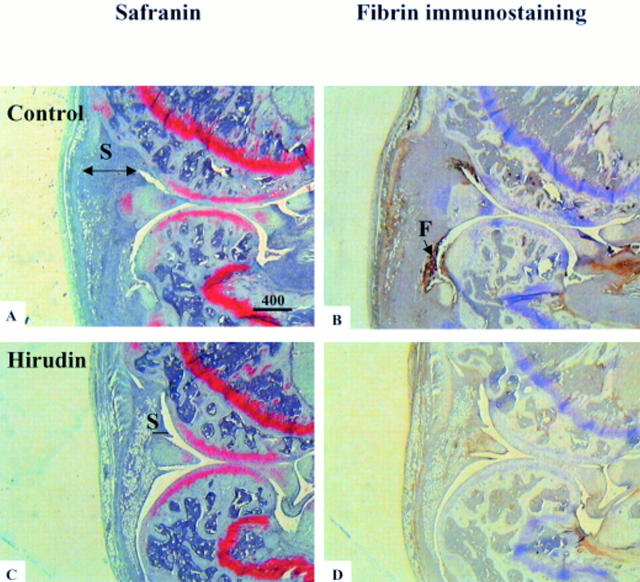
Histologies and immunohistologies of whole knee joint sections of control and of hirudin treated mice with antigen induced arthritis. Figures A and C show safranin O stained sections of arthritic knee joints at day 10 after arthritis induction. Note the difference of thickness of synovial membrane (S) which is thicker in the control arthritic mice than in the hirudin treated ones. Figures B and C show fibrin(ogen) immunostaining (indicated by (F) on Figure 3B) of adjacent sections.
Figure 4 .
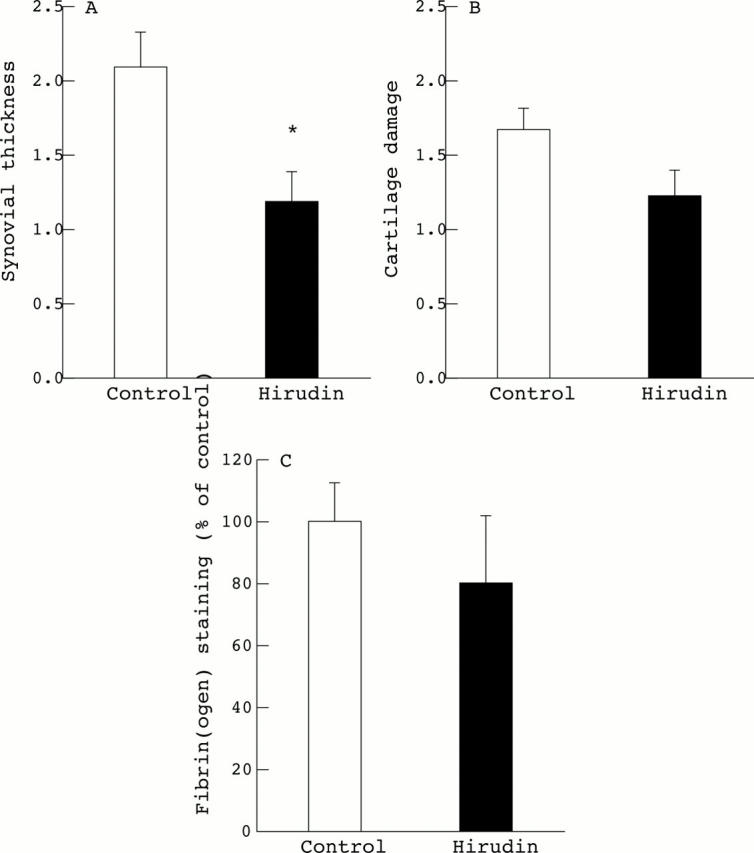
Histological grading and fibrin scoring of arthritic knee joints. Synovial thickness (A) and cartilage damage (B) were scored histologically using an arbitrary scale from 0 to 3. Fibrin deposition (C) was scored in the synovial membrane using an arbitrary scale from 0 to 6 and expressed as a percentage of fibrin deposition (control = 100%). Results are expressed as means (SEM), control and hirudin treated mice, n=11 per group. Statistical significance was tested by Wilcoxon/Kruskal-Wallis tests (rank sums). *p<0.05 was considered significant.
Figure 5 .
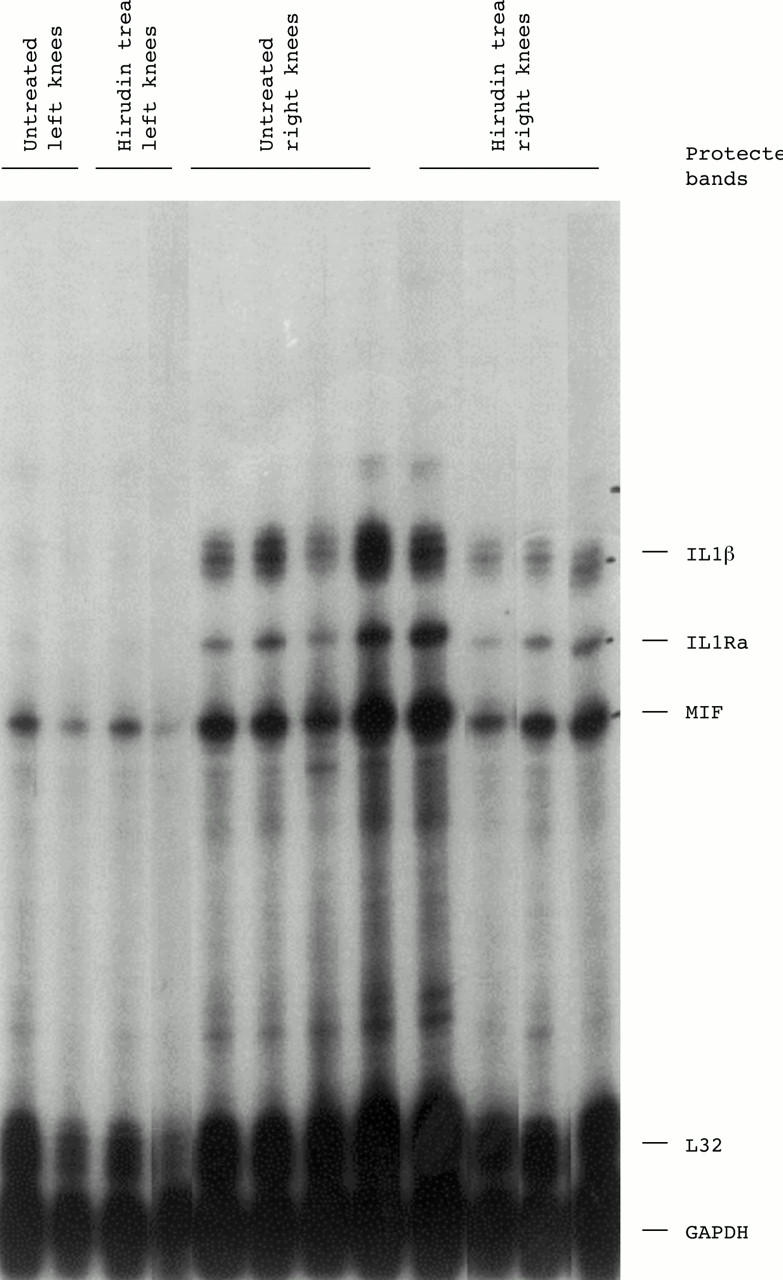
Synovial cytokine mRNA levels in knee joints from untreated or hirudin treated mice. Total RNA was prepared from non-arthritic (left knee) or from arthritic (right knee) joints of untreated or hirudin treated mice at day 10 after induction of arthritis. Expression of different cytokine mRNAs was analysed by RNase protection assay using a multiprobe set which included interleukin 1β (IL1β), IL1 receptor antagonist (IL1Ra), and macrophage migration inhibitory factor (MIF).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Busso N., Péclat V., Van Ness K., Kolodziesczyk E., Degen J., Bugge T., So A. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998 Jul 1;102(1):41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmassi F., de Negri F., Morale M., Song K. Y., Chung S. I. Fibrin degradation in the synovial fluid of rheumatoid arthritis patients: a model for extravascular fibrinolysis. Semin Thromb Hemost. 1996;22(6):489–496. doi: 10.1055/s-2007-999049. [DOI] [PubMed] [Google Scholar]
- Cicala C., Cirino G. Linkage between inflammation and coagulation: an update on the molecular basis of the crosstalk. Life Sci. 1998;62(20):1817–1824. doi: 10.1016/s0024-3205(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Cirino G., Cicala C., Bucci M. R., Sorrentino L., Maraganore J. M., Stone S. R. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996 Mar 1;183(3):821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmensen I., Hølund B., Andersen R. B. Fibrin and fibronectin in rheumatoid synovial membrane and rheumatoid synovial fluid. Arthritis Rheum. 1983 Apr;26(4):479–485. doi: 10.1002/art.1780260405. [DOI] [PubMed] [Google Scholar]
- Connolly A. J., Ishihara H., Kahn M. L., Farese R. V., Jr, Coughlin S. R. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996 Jun 6;381(6582):516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R. Sol Sherry lecture in thrombosis: how thrombin 'talks' to cells: molecular mechanisms and roles in vivo. Arterioscler Thromb Vasc Biol. 1998 Apr;18(4):514–518. doi: 10.1161/01.atv.18.4.514. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Senger D. R., Dvorak A. M., Harvey V. S., McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985 Mar 1;227(4690):1059–1061. doi: 10.1126/science.3975602. [DOI] [PubMed] [Google Scholar]
- Fareed J., Callas D., Hoppensteadt D. A., Lewis B. E., Bick R. L., Walenga J. M. Antithrombin agents as anticoagulants and antithrombotics: implications in drug development. Semin Hematol. 1999 Jan;36(1 Suppl 1):42–56. [PubMed] [Google Scholar]
- Furmaniak-Kazmierczak E., Cooke T. D., Manuel R., Scudamore A., Hoogendorn H., Giles A. R., Nesheim M. Studies of thrombin-induced proteoglycan release in the degradation of human and bovine cartilage. J Clin Invest. 1994 Aug;94(2):472–480. doi: 10.1172/JCI117358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijsen M. W., van den Berg W. B., van de Putte L. B., van den Broek W. J. Detection and quantification of experimental joint inflammation in mice by measurement of 99mTc-pertechnetate uptake. Agents Actions. 1981 Dec;11(6-7):640–642. doi: 10.1007/BF01978775. [DOI] [PubMed] [Google Scholar]
- Mapp P. I., Grootveld M. C., Blake D. R. Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull. 1995 Apr;51(2):419–436. doi: 10.1093/oxfordjournals.bmb.a072970. [DOI] [PubMed] [Google Scholar]
- Markwardt F. The development of hirudin as an antithrombotic drug. Thromb Res. 1994 Apr 1;74(1):1–23. doi: 10.1016/0049-3848(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Morris R., Winyard P. G., Blake D. R., Morris C. J. Thrombin in inflammation and healing: relevance to rheumatoid arthritis. Ann Rheum Dis. 1994 Jan;53(1):72–79. doi: 10.1136/ard.53.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R., Winyard P. G., Brass L. F., Blake D. R., Morris C. J. Thrombin receptor expression in rheumatoid and osteoarthritic synovial tissue. Ann Rheum Dis. 1996 Nov;55(11):841–843. doi: 10.1136/ard.55.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S., Ikata T., Kinoshita I., Kanematsu J., Yasuoka S. Characteristics of the protease activity in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Exp Rheumatol. 1999 Mar-Apr;17(2):161–170. [PubMed] [Google Scholar]
- Ohba T., Takase Y., Ohhara M., Kasukawa R. Thrombin in the synovial fluid of patients with rheumatoid arthritis mediates proliferation of synovial fibroblast-like cells by induction of platelet derived growth factor. J Rheumatol. 1996 Sep;23(9):1505–1511. [PubMed] [Google Scholar]
- Perez R. L., Roman J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995 Feb 15;154(4):1879–1887. [PubMed] [Google Scholar]
- Qi J., Kreutzer D. L. Fibrin activation of vascular endothelial cells. Induction of IL-8 expression. J Immunol. 1995 Jul 15;155(2):867–876. [PubMed] [Google Scholar]
- Robson S. C., Shephard E. G., Kirsch R. E. Fibrin degradation product D-dimer induces the synthesis and release of biologically active IL-1 beta, IL-6 and plasminogen activator inhibitors from monocytes in vitro. Br J Haematol. 1994 Feb;86(2):322–326. doi: 10.1111/j.1365-2141.1994.tb04733.x. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Skogen W. F., Griffin G. L., Wilner G. D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986 Mar;77(3):1014–1019. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Nakajima T., Kitajima I., Shigeta K., Abeyama K., Imamura T., Okano T., Kawahara K., Nakamura T., Maruyama I. Thrombin receptor-mediated synovial proliferation in patients with rheumatoid arthritis. Clin Immunol Immunopathol. 1995 Sep;76(3 Pt 1):225–233. doi: 10.1006/clin.1995.1120. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Pippen A. M., Greenberg C. S. Extravascular fibrin formation and dissolution in synovial tissue of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum. 1991 Aug;34(8):996–1005. doi: 10.1002/art.1780340809. [DOI] [PubMed] [Google Scholar]
- Zacharski L. R., Brown F. E., Memoli V. A., Kisiel W., Kudryk B. J., Rousseau S. M., Hunt J. A., Dunwiddie C., Nutt E. M. Pathways of coagulation activation in situ in rheumatoid synovial tissue. Clin Immunol Immunopathol. 1992 May;63(2):155–162. doi: 10.1016/0090-1229(92)90008-c. [DOI] [PubMed] [Google Scholar]