Abstract
OBJECTIVES—To investigate whether apoptosis occurs in osteoarthritis (OA), and if this phenomenon is modulated by human recombinant interleukin 1β (hrIL1β). METHODS—Human articular cartilage samples were obtained at the time of hip arthroplasty because of femoral neck fracture (normal cartilage) (n=4) or advanced coxarthrosis (OA cartilage) (n=14). Apoptotic chondrocytes, isolated by collagenase digestion and cultivated for 24 hours, or present in situ in frozen cartilage sections, were quantified by fluorescent microscopy using two apoptosis markers: the TUNEL reaction, which detects nuclear DNA fragmentation, and Annexin-V-fluos, which labels at the membrane level the externalisation of phosphatidylserine. RESULTS—In OA cartilage 18-21% of chondrocytes showed apoptotic features, compared with 2-5% in normal cartilage. The results were similar for the two comparative studies (in situ and in vitro) and for both apoptosis markers. Moreover, hrIL1β increased the apoptosis rate in vitro in a dose dependent manner in OA and normal chondrocytes. CONCLUSION—These results suggest that apoptosis may be an important factor in the evolution of OA and may be a new target for treatment of OA.
Full Text
The Full Text of this article is available as a PDF (183.4 KB).
Figure 1 .
Detection of apoptosis by immunofluorescence on isolated OA chondrocytes by collagenase digestion (A and C) and in situ on fresh frozen OA cartilage (B and D). (A and B) Chondrocytes were fixed, permeabilised, and processed by the TUNEL reaction mixture. (C and D) Samples were treated with Annexin-V-fluos for 15 minutes and analysed immediately. An example of an apoptotic cell is identified by the arrows. (Original magnification A, B, C ×200; D ×100).
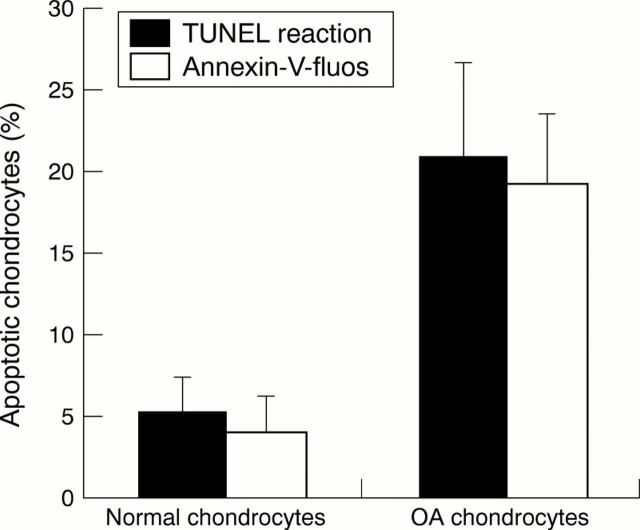
Figure 2 .
Comparative study of normal and OA chondrocytes using the TUNEL reaction and Annexin-V labelling in chondrocytes isolated by collagenase digestion. The ratio of apoptotic cells/total cells was calculated as a percentage. Values are means (SEM) from four experiments for normal chondrocytes and 11 experiments for OA chondrocytes (p<0.01).
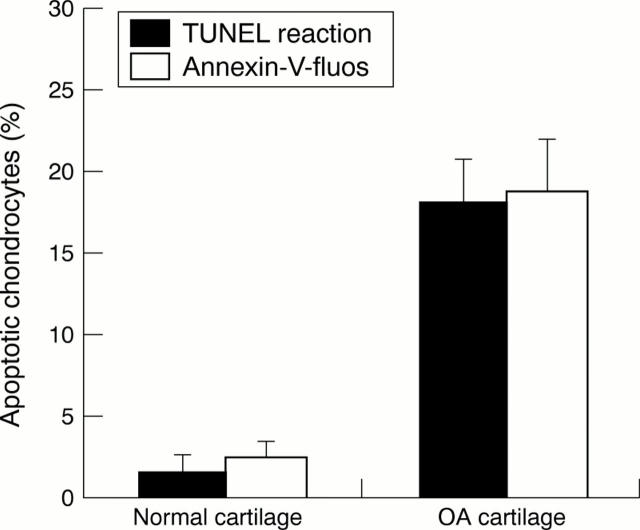
Figure 3 .
Comparative study of normal and OA cartilage using the TUNEL reaction and Annexin-V labelling on frozen cartilage sections. The ratio of apoptotic cells/total cells was calculated as a percentage. Data are representative results from four experiments with normal cartilage and 13 experiments with OA cartilage (p<0.01).
Figure 4 .
Induction of apoptosis in OA chondrocytes by human recombinant interleukin 1β (hrIL1β). (A) Untreated control OA human chondrocytes. (B) OA human articular chondrocytes in primary culture stimulated with 10 ng/ml of hrIL1β for 24 hours and processed with Annexin-V-fluos for 15 minutes. Arrows identify examples of apoptotic cells. (Original magnification ×200.)
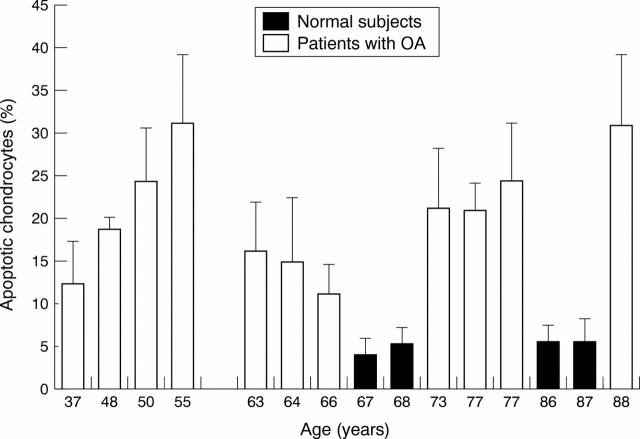
Figure 5 .
Percentage of apoptotic chondrocytes as a function of aging in the patients with OA and normal patients. Cells were isolated by collagenase digestion, and apoptosis in these isolated cells was analysed by TUNEL reaction labelling. Values are means (SEM) for 11 patients with OA and four normal patients.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. S., Horton W. E., Jr Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998 Apr;250(4):418–425. doi: 10.1002/(SICI)1097-0185(199804)250:4<418::AID-AR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Aizawa T., Kokubun S., Tanaka Y. Apoptosis and proliferation of growth plate chondrocytes in rabbits. J Bone Joint Surg Br. 1997 May;79(3):483–486. doi: 10.1302/0301-620x.79b3.7221. [DOI] [PubMed] [Google Scholar]
- Altman R., Alarcón G., Appelrouth D., Bloch D., Borenstein D., Brandt K., Brown C., Cooke T. D., Daniel W., Feldman D. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991 May;34(5):505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Guitian R., Vázquez-Martul E., de Toro F. J., Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum. 1998 Feb;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Blanco F. J., Ochs R. L., Schwarz H., Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol. 1995 Jan;146(1):75–85. [PMC free article] [PubMed] [Google Scholar]
- Bosman F. T., Visser B. C., van Oeveren J. Apoptosis: pathophysiology of programmed cell death. Pathol Res Pract. 1996 Jul;192(7):676–683. doi: 10.1016/S0344-0338(96)80089-6. [DOI] [PubMed] [Google Scholar]
- Cooper C., Inskip H., Croft P., Campbell L., Smith G., McLaren M., Coggon D. Individual risk factors for hip osteoarthritis: obesity, hip injury, and physical activity. Am J Epidemiol. 1998 Mar 15;147(6):516–522. doi: 10.1093/oxfordjournals.aje.a009482. [DOI] [PubMed] [Google Scholar]
- Evans C. H. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995;47:107–116. doi: 10.1007/978-3-0348-7343-7_9. [DOI] [PubMed] [Google Scholar]
- Felson D. T., Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum. 1998 Aug;41(8):1343–1355. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G., Lin D. L., Roque M. Apoptosis of terminally differentiated chondrocytes in culture. Exp Cell Res. 1997 Jun 15;233(2):372–382. doi: 10.1006/excr.1997.3576. [DOI] [PubMed] [Google Scholar]
- Grabowski P. S., Macpherson H., Ralston S. H. Nitric oxide production in cells derived from the human joint. Br J Rheumatol. 1996 Mar;35(3):207–212. doi: 10.1093/rheumatology/35.3.207. [DOI] [PubMed] [Google Scholar]
- Hamerman D. Clinical implications of osteoarthritis and ageing. Ann Rheum Dis. 1995 Feb;54(2):82–85. doi: 10.1136/ard.54.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmand M. F., Duphil R., Blanquet P. Proteoglycan synthesis in chondrocyte cultures from osteoarthrotic and normal human articular cartilage. Biochim Biophys Acta. 1982 Aug 6;717(2):190–202. doi: 10.1016/0304-4165(82)90169-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Ochs R. L., Komiya S., Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 1998 Sep;41(9):1632–1638. doi: 10.1002/1529-0131(199809)41:9<1632::AID-ART14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Setareh M., Ochs R. L., Lotz M. Fas/Fas ligand expression and induction of apoptosis in chondrocytes. Arthritis Rheum. 1997 Oct;40(10):1749–1755. doi: 10.1002/art.1780401004. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Takahashi K., Amiel D., Coutts R. D., Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998 Jul;41(7):1266–1274. doi: 10.1002/1529-0131(199807)41:7<1266::AID-ART18>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Abe E., Yamate T., Taguchi Y., Jasin H. E. Nitric oxide production by superficial and deep articular chondrocytes. Arthritis Rheum. 1997 Feb;40(2):261–269. doi: 10.1002/art.1780400210. [DOI] [PubMed] [Google Scholar]
- Hoa T. T., Hasunuma T., Aono H., Masuko K., Kobata T., Yamamoto K., Sumida T., Nishioka K. Novel mechanisms of selective apoptosis in synovial T cells of patients with rheumatoid arthritis. J Rheumatol. 1996 Aug;23(8):1332–1337. [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Hashimoto S., Kühn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999 Jul;7(4):389–391. doi: 10.1053/joca.1998.0220. [DOI] [PubMed] [Google Scholar]
- Maier R., Bilbe G., Rediske J., Lotz M. Inducible nitric oxide synthase from human articular chondrocytes: cDNA cloning and analysis of mRNA expression. Biochim Biophys Acta. 1994 Sep 21;1208(1):145–150. doi: 10.1016/0167-4838(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Martel-Pelletier J., McCollum R., DiBattista J., Faure M. P., Chin J. A., Fournier S., Sarfati M., Pelletier J. P. The interleukin-1 receptor in normal and osteoarthritic human articular chondrocytes. Identification as the type I receptor and analysis of binding kinetics and biologic function. Arthritis Rheum. 1992 May;35(5):530–540. doi: 10.1002/art.1780350507. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Reutelingsperger C. P., McGahon A. J., Rader J. A., van Schie R. C., LaFace D. M., Green D. R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995 Nov 1;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Müller-Ladner U., Gay R. E., Nishioka K., Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J Rheumatol. 1996 Aug;23(8):1345–1352. [PubMed] [Google Scholar]
- Mollenhauer J., Mok M. T., King K. B., Gupta M., Chubinskaya S., Koepp H., Cole A. A. Expression of anchorin CII (cartilage annexin V) in human young, normal adult, and osteoarthritic cartilage. J Histochem Cytochem. 1999 Feb;47(2):209–220. doi: 10.1177/002215549904700209. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Aono H., Hasunuma T., Yamamoto K., Shirai T., Hirohata K., Nishioka K. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995 Apr;38(4):485–491. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- Oliveria S. A., Felson D. T., Cirillo P. A., Reed J. I., Walker A. M. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999 Mar;10(2):161–166. [PubMed] [Google Scholar]
- Palmer R. M., Hickery M. S., Charles I. G., Moncada S., Bayliss M. T. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993 May 28;193(1):398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- Taskiran D., Stefanovic-Racic M., Georgescu H., Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994 Apr 15;200(1):142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- Vignon E., Arlot M., Meunier P., Vignon G. Quantitative histological changes in osteoarthritic hip cartilage. Morphometric analysis of 29 osteoarthritic and 26 normal human femoral heads. Clin Orthop Relat Res. 1974;(103):269–278. [PubMed] [Google Scholar]
- Vignon E., Arlot M., Patricot L. M., Vignon G. The cell density of human femoral head cartilage. Clin Orthop Relat Res. 1976 Nov-Dec;(121):303–308. [PubMed] [Google Scholar]
- Vingård E. Overweight predisposes to coxarthrosis. Body-mass index studied in 239 males with hip arthroplasty. Acta Orthop Scand. 1991 Apr;62(2):106–109. doi: 10.3109/17453679108999233. [DOI] [PubMed] [Google Scholar]