Abstract
OBJECTIVE—To determine whether tissue angiotensin converting enzyme (ACE) is increased in synovia from patients with rheumatoid arthritis, osteoarthritis or chondromalacia patellae. METHODS—Sections of synovia from patients with rheumatoid arthritis (n = 7), osteoarthritis (n = 7) or chondromalacia patellae (n = 6) were tested for immunoreactivity for ACE, and for binding of the ACE inhibitor [125I]351A. The amount of ACE was measured with computer assisted image analysis as the proportion of synovial section area occupied by ACE-immunoreactive cells, and the density of [125I]351A binding. RESULTS—[125I]351A binding sites had characteristics of ACE and colocalised with ACE-like immunoreactivity to microvascular endothelium and fibroblast-like stromal cells in inflamed and non-inflamed human synovium. Stromal [125I]351A binding densities (Beq) and the fraction of synovial section area occupied by ACE-immunoreactivity (fractional area) were higher in synovia from patients with rheumatoid arthritis (Beq 28 amol/mm2, fractional area 0.21) than from those with osteoarthritis (Beq 9 amol/mm2, fractional area 0.10) or chondromalacia patellae (Beq 9 amol/mm2, fractional area 0.09)(p < 0.05). Density of [125I]351A binding to stroma was similar to that to blood vessels in rheumatoid arthritis, but less dense than vascular binding in chondromalacia patellae and osteoarthritis. Increases in [125I]351A binding densities were attributable to increases in the numbers of binding sites, and were consistent with an increase in the density of ACE bearing stromal cells. CONCLUSION—ACE is upregulated in synovial stroma in rheumatoid arthritis. Increased tissue ACE may result in increased local generation of the vasoconstrictor and mitogenic peptide angiotensin II and thereby potentiate synovial hypoxia and proliferation in rheumatoid arthritis.
Full Text
The Full Text of this article is available as a PDF (190.1 KB).
Figure 1 .
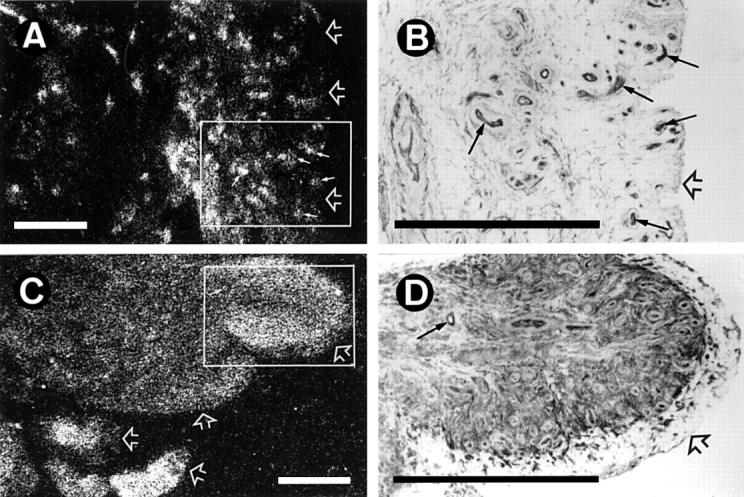
Localisation of [125I]351A binding sites and angiotensin converting enzyme-like immunoreactivity (ACE-LI) in human synovium. (A) [125I]351A binding to synovium from a patient with chondromalacia patellae showing punctate binding to blood vessels (small arrows), with less dense binding to stromal tissue between vessels. (B) ACE-LI in the field of a consecutive tissue section that corresponds to the box shown in (A). Small arrows indicate ACE-LI localised to vascular endothelium corresponding to the punctate [125I]351A binding in (A). Less intense ACE-LI is localised to spindle shaped cells within the synovial stroma. (C) [125I]351A binding to synovium from a patient with rheumatoid arthritis showing diffuse stromal binding of a similar density to punctate binding (small arrow). (D) ACE-LI in the field of a consecutive tissue section that corresponds to the box shown in (C). Intense ACE-LI is localised to stromal cells, particularly those immediately beneath the lining cells and around blood vessels. The small arrow indicates ACE-LI localised to vascular endothelium corresponding to punctate binding in (C). (A) and (C) Reversal prints of film autoradiograms. (B) and (D) Immunohistochemistry with rabbit polyclonal antihuman ACE, developed with DAB using glucose oxidase/nickel enhancement. Open arrows indicate synovial lining region. Bars = 500 µm.
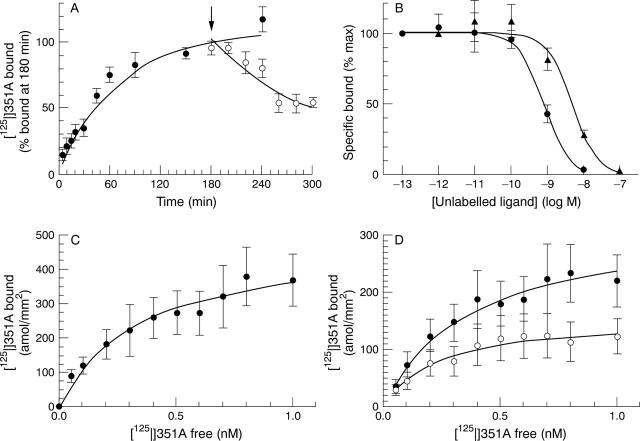
Figure 2 .
Characteristics of [125I]351A binding to human synovium. (A) Association (filled circles) and dissociation (empty circles) at 22°C of 0.03 nM [125I]351A binding to blood vessels in synovia from patients with rheumatoid arthritis. For dissociation time course, sections were transferred to an excess of buffer without [125I]351A at 180 minutes (arrow). (B) Inhibition of 0.03 nM [125I]351A binding to blood vessels in synovia from patients with rheumatoid arthritis by unlabelled lisinopril (circles) or captopril (triangles). (C) Saturation of [125I]351A binding to blood vessels in synovia from patients with rheumatoid arthritis. (D) Saturation of [125I]351A binding to synovial stroma from patients with rheumatoid arthritis (filled circles) or osteoarthritis (empty circles). Points represent means (SEM) for synovia from six to eight patients.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R., Asch E., Bloch D., Bole G., Borenstein D., Brandt K., Christy W., Cooke T. D., Greenwald R., Hochberg M. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986 Aug;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bathon J. M., Manning D. C., Goldman D. W., Towns M. C., Proud D. Characterization of kinin receptors on human synovial cells and upregulation of receptor number by interleukin-1. J Pharmacol Exp Ther. 1992 Jan;260(1):384–392. [PubMed] [Google Scholar]
- Bird H. A., Le Gallez P., Dixon J. S., Catalano M. A., Traficante A., Liauw L. A., Sussman H., Rotman H., Wright V. A clinical and biochemical assessment of a nonthiol ACE inhibitor (pentopril; CGS-13945) in active rheumatoid arthritis. J Rheumatol. 1990 May;17(5):603–608. [PubMed] [Google Scholar]
- Brilla C. G., Zhou G., Matsubara L., Weber K. T. Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol. 1994 Jul;26(7):809–820. doi: 10.1006/jmcc.1994.1098. [DOI] [PubMed] [Google Scholar]
- Caspritz G., Alpermann H. G., Schleyerbach R. Influence of the new angiotensin converting enzyme inhibitor ramipril on several models of acute inflammation and the adjuvant arthritis in the rat. Arzneimittelforschung. 1986 Nov;36(11):1605–1608. [PubMed] [Google Scholar]
- Catt K. J., Baukal A. Prolonged retention of high specific activity by 125I-labeled angiotensin II--a consequence of 'decay catastrophe'. Biochim Biophys Acta. 1973 Jun 20;313(1):221–225. doi: 10.1016/0304-4165(73)90203-1. [DOI] [PubMed] [Google Scholar]
- Challah M., Nicoletti A., Arnal J. F., Philippe M., Laboulandine I., Allegrini J., Alhenc-Gelas F., Danilov S., Michel J. B. Cardiac angiotensin converting enzyme overproduction indicates interstitial activation in renovascular hypertension. Cardiovasc Res. 1995 Aug;30(2):231–239. [PubMed] [Google Scholar]
- Correa F. M., Guilhaume S. S., Saavedra J. M. Comparative quantification of rat brain and pituitary angiotensin-converting enzyme with autoradiographic and enzymatic methods. Brain Res. 1991 Apr 5;545(1-2):215–222. doi: 10.1016/0006-8993(91)91289-d. [DOI] [PubMed] [Google Scholar]
- Fan T. P., Hu D. E., Guard S., Gresham G. A., Watling K. J. Stimulation of angiogenesis by substance P and interleukin-1 in the rat and its inhibition by NK1 or interleukin-1 receptor antagonists. Br J Pharmacol. 1993 Sep;110(1):43–49. doi: 10.1111/j.1476-5381.1993.tb13769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. A., Absher M. Norepinephrine and ANG II stimulate secretion of TGF-beta by neonatal rat cardiac fibroblasts in vitro. Am J Physiol. 1995 Apr;268(4 Pt 1):C910–C917. doi: 10.1152/ajpcell.1995.268.4.C910. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Fyhrquist F., Tikkanen I., Grönhagen-Riska C., Hortling L., Hichens M. Inhibitor binding assay for angiotensin-converting enzyme. Clin Chem. 1984 May;30(5):696–700. [PubMed] [Google Scholar]
- Hafizi S., Wharton J., Morgan K., Allen S. P., Chester A. H., Catravas J. D., Polak J. M., Yacoub M. H. Expression of functional angiotensin-converting enzyme and AT1 receptors in cultured human cardiac fibroblasts. Circulation. 1998 Dec 8;98(23):2553–2559. doi: 10.1161/01.cir.98.23.2553. [DOI] [PubMed] [Google Scholar]
- Howell S., Brewis I. A., Hooper N. M., Kenny A. J., Turner A. J. Mosaic expression of membrane peptidases by confluent cultures of Caco-2 cells. FEBS Lett. 1993 Feb 8;317(1-2):109–112. doi: 10.1016/0014-5793(93)81502-q. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Izai M., Miyazaki S., Murai R., Morioka Y., Hayashi H., Nishiura M., Miura K. Prorenin-renin axis in synovial fluid in patients with rheumatoid arthritis and osteoarthritis. Endocrinol Jpn. 1992 Jun;39(3):259–267. doi: 10.1507/endocrj1954.39.259. [DOI] [PubMed] [Google Scholar]
- Katwa L. C., Campbell S. E., Tyagi S. C., Lee S. J., Cicila G. T., Weber K. T. Cultured myofibroblasts generate angiotensin peptides de novo. J Mol Cell Cardiol. 1997 May;29(5):1375–1386. doi: 10.1006/jmcc.1997.0376. [DOI] [PubMed] [Google Scholar]
- Lowe J. R., Dixon J. S., Guthrie J. A., McWhinney P. Serum and synovial fluid levels of angiotensin converting enzyme in polyarthritis. Ann Rheum Dis. 1986 Nov;45(11):921–924. doi: 10.1136/ard.45.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. F., Surrall K. E., McKenna F., Dixon J. S., Bird H. A., Wright V. Captopril: a new treatment for rheumatoid arthritis? Lancet. 1984 Jun 16;1(8390):1325–1328. doi: 10.1016/s0140-6736(84)91821-x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A. Localization of angiotensin converting enzyme in rat forebrain and other tissues by in vitro autoradiography using 125I-labelled MK351A. Clin Exp Pharmacol Physiol. 1984 Jul-Aug;11(4):431–435. doi: 10.1111/j.1440-1681.1984.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Metsärinne K. P., Nordström D. C., Konttinen Y. T., Teppo A. M., Fyhrquist F. Y. Plasma interleukin-6 and renin substrate in reactive arthritis, rheumatoid arthritis, and systemic lupus erythematosus. Rheumatol Int. 1992;12(3):93–96. doi: 10.1007/BF00290261. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Rubin D. B., Mason R. J., Dobbs L. J. Angiotensin-converting enzyme substrates hydrolyzed by fibroblasts and vascular endothelial cells. Exp Lung Res. 1982 May;3(2):137–145. doi: 10.3109/01902148209063288. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993 Sep;73(3):413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- Shu S. Y., Ju G., Fan L. Z. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988 Feb 29;85(2):169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Smits J. F., van Krimpen C., Schoemaker R. G., Cleutjens J. P., Daemen M. J. Angiotensin II receptor blockade after myocardial infarction in rats: effects on hemodynamics, myocardial DNA synthesis, and interstitial collagen content. J Cardiovasc Pharmacol. 1992;20(5):772–778. [PubMed] [Google Scholar]
- Subissi A., Guelfi M., Criscuoli M. Angiotensin converting enzyme inhibitors potentiate the bronchoconstriction induced by substance P in the guinea-pig. Br J Pharmacol. 1990 Jul;100(3):502–506. doi: 10.1111/j.1476-5381.1990.tb15837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Cleutjens J. P., Diaz-Arias A. A., Weber K. T. Cardiac angiotensin converting enzyme and myocardial fibrosis in the rat. Cardiovasc Res. 1994 Sep;28(9):1423–1432. doi: 10.1093/cvr/28.9.1423. [DOI] [PubMed] [Google Scholar]
- Sun Y., Weber K. T. Angiotensin-converting enzyme and wound healing in diverse tissues of the rat. J Lab Clin Med. 1996 Jan;127(1):94–101. doi: 10.1016/s0022-2143(96)90170-5. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Unger T., Gohlke P. Tissue renin-angiotensin systems in the heart and vasculature: possible involvement in the cardiovascular actions of converting enzyme inhibitors. Am J Cardiol. 1990 May 22;65(19):3I–10I. doi: 10.1016/0002-9149(90)90118-k. [DOI] [PubMed] [Google Scholar]
- Veale D., Yanni G., Bresnihan B., FitzGerald O. Production of angiotensin converting enzyme by rheumatoid synovial membrane. Ann Rheum Dis. 1992 Apr;51(4):476–480. doi: 10.1136/ard.51.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert O. V., Ward W. F., Lingen M. W., Chesler L., Solt D. B., Johnson M. D., Molteni A., Polverini P. J., Bouck N. P. Captopril inhibits angiogenesis and slows the growth of experimental tumors in rats. J Clin Invest. 1996 Aug 1;98(3):671–679. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Hu D. E., Wharton J., Catravas J. D., Blake D. R., Fan T. P. Sequential development of angiotensin receptors and angiotensin I converting enzyme during angiogenesis in the rat subcutaneous sponge granuloma. Br J Pharmacol. 1997 Apr;120(7):1302–1311. doi: 10.1038/sj.bjp.0701062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Mapp P. I., Wharton J., Polak J. M., Blake D. R. Neuropeptide degrading enzymes in normal and inflamed human synovium. Am J Pathol. 1993 May;142(5):1610–1621. [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Salmon M., Mapp P. I., Wharton J., Garrett N., Blake D. R., Polak J. M. Microvascular substance P binding to normal and inflamed rat and human synovium. J Pharmacol Exp Ther. 1993 Nov;267(2):951–960. [PubMed] [Google Scholar]
- Walsh D. A., Suzuki T., Knock G. A., Blake D. R., Polak J. M., Wharton J. AT1 receptor characteristics of angiotensin analogue binding in human synovium. Br J Pharmacol. 1994 Jun;112(2):435–442. doi: 10.1111/j.1476-5381.1994.tb13091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A., Wade M., Mapp P. I., Blake D. R. Focally regulated endothelial proliferation and cell death in human synovium. Am J Pathol. 1998 Mar;152(3):691–702. [PMC free article] [PubMed] [Google Scholar]
- Weinberg K. S., Douglas W. H., MacNamee D. R., Lanzillo J. J., Fanburg B. L. Angiotensin I-converting enzyme localization on cultured fibroblasts by immunofluorescence. In Vitro. 1982 Apr;18(4):400–406. doi: 10.1007/BF02796341. [DOI] [PubMed] [Google Scholar]
- Wharton J., Morgan K., Rutherford R. A., Catravas J. D., Chester A., Whitehead B. F., De Leval M. R., Yacoub M. H., Polak J. M. Differential distribution of angiotensin AT2 receptors in the normal and failing human heart. J Pharmacol Exp Ther. 1998 Jan;284(1):323–336. [PubMed] [Google Scholar]
- Wu L. L., Yang N., Roe C. J., Cooper M. E., Gilbert R. E., Atkins R. C., Lan H. Y. Macrophage and myofibroblast proliferation in remnant kidney: role of angiotensin II. Kidney Int Suppl. 1997 Dec;63:S221–S225. [PubMed] [Google Scholar]
- van Krimpen C., Smits J. F., Cleutjens J. P., Debets J. J., Schoemaker R. G., Struyker Boudier H. A., Bosman F. T., Daemen M. J. DNA synthesis in the non-infarcted cardiac interstitium after left coronary artery ligation in the rat: effects of captopril. J Mol Cell Cardiol. 1991 Nov;23(11):1245–1253. doi: 10.1016/0022-2828(91)90082-w. [DOI] [PubMed] [Google Scholar]