Abstract
OBJECTIVE—Previously an upregulation of E-cadherin and its associated molecules α-catenin, β-catenin and plakoglobin has been demonstrated in clinically overt inflammatory bowel disease (IBD). The aim of this study was to investigate the expression of the E-cadherin/catenin complex in subclinically inflamed bowel mucosa from spondyloarthropathy (SpA) patients. METHODS—Ileal and colonic biopsy specimens from 19 SpA patients with subclinical inflammatory gut lesions and from seven controls were stained with monoclonal antibodies against E-cadherin, β-catenin and plakoglobin and a polyclonal antibody against α-catenin. E-cadherin mRNA was detected using a riboprobe. Inflammation was histologically classified into acute, chronic active and chronic quiescent forms. RESULTS—In acute and chronic active bowel inflammation of SpA patients, upregulation of the E-cadherin/catenin glycoprotein complex could be observed. Chronic lesions in a quiescent state did not show such an upregulation. Furthermore, chronic inflammation was associated with an increase in E-cadherin mRNA. CONCLUSIONS—As some of the SpA patients with subclinical gut inflammation develop IBD, upregulation of the E-cadherin/catenin complex in inflamed bowel mucosa from SpA patients may point to early cellular changes in the development of IBD. However, at present it cannot be excluded that increased E-cadherin/catenin complex expression is a bystander phenomenon of active inflammation.
Full Text
The Full Text of this article is available as a PDF (222.2 KB).
Figure 1 .
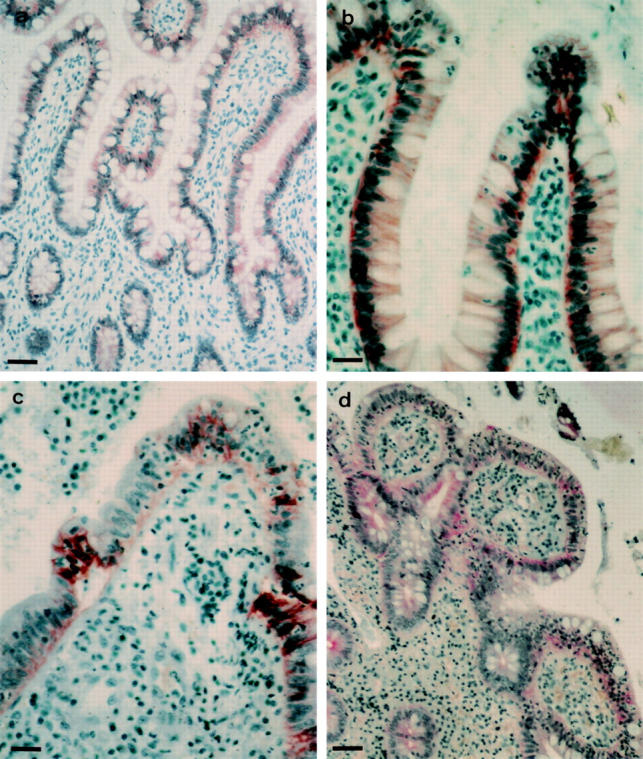
E-cadherin/catenin immunoreactivity in normal and subclinically inflamed bowel mucosa. (a): Weak α-catenin expression in non-inflamed ileum. (b): Acute ileitis showing strong expression of α-catenin in all cells of the villi. (c): Focal upregulation of E-cadherin in villus epithelial cells in chronic active ileitis. (d): β-catenin immunoreactivity in villus and crypt cells in chronic active ileitis. (a) and (c) Bar = 40 µm; (b) and (d) bar = 25 µm.
Figure 2 .
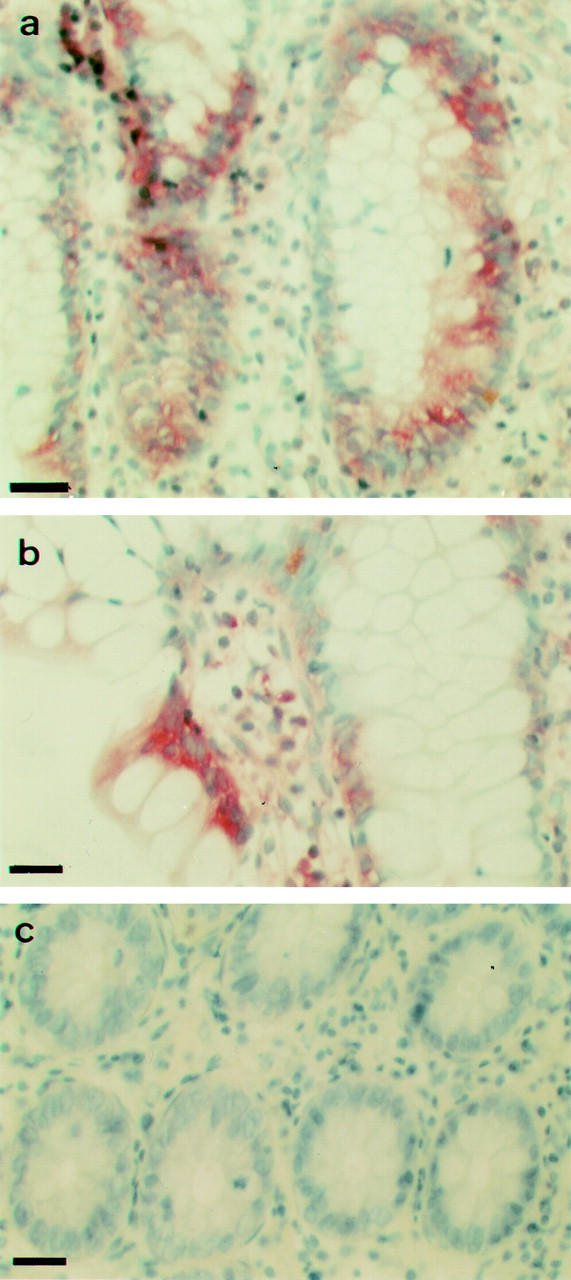
E-cadherin mRNA in situ hybridisation. (a): Strong positivity in chronic quiescent ileitis. (b): Focal upregulation in chronic quiescent ileitis. (c): Absence of detectable expression in normal colon. (a), (b) and (c) Bar = 25 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Bailey C. J., Hembry R. M., Alexander A., Irving M. H., Grant M. E., Shuttleworth C. A. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn's disease and normal intestine. J Clin Pathol. 1994 Feb;47(2):113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracke M. E., Van Roy F. M., Mareel M. M. The E-cadherin/catenin complex in invasion and metastasis. Curr Top Microbiol Immunol. 1996;213(Pt 1):123–161. doi: 10.1007/978-3-642-61107-0_9. [DOI] [PubMed] [Google Scholar]
- Bussemakers M. J., van Bokhoven A., Mees S. G., Kemler R., Schalken J. A. Molecular cloning and characterization of the human E-cadherin cDNA. Mol Biol Rep. 1993 Feb;17(2):123–128. doi: 10.1007/BF00996219. [DOI] [PubMed] [Google Scholar]
- Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994 Nov 10;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Cuvelier C., Barbatis C., Mielants H., De Vos M., Roels H., Veys E. Histopathology of intestinal inflammation related to reactive arthritis. Gut. 1987 Apr;28(4):394–401. doi: 10.1136/gut.28.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier C., Mielants H., De Vos M., Veys E., Roels H. Major histocompatibility complex class II antigen (HLA-DR) expression by ileal epithelial cells in patients with seronegative spondylarthropathy. Gut. 1990 May;31(5):545–549. doi: 10.1136/gut.31.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M., Cuvelier C., Mielants H., Veys E., Barbier F., Elewaut A. Ileocolonoscopy in seronegative spondylarthropathy. Gastroenterology. 1989 Feb;96(2 Pt 1):339–344. doi: 10.1016/0016-5085(89)91557-6. [DOI] [PubMed] [Google Scholar]
- De Vos M., Mielants H., Cuvelier C., Elewaut A., Veys E. Long-term evolution of gut inflammation in patients with spondyloarthropathy. Gastroenterology. 1996 Jun;110(6):1696–1703. doi: 10.1053/gast.1996.v110.pm8964393. [DOI] [PubMed] [Google Scholar]
- Dougados M., van der Linden S., Juhlin R., Huitfeldt B., Amor B., Calin A., Cats A., Dijkmans B., Olivieri I., Pasero G. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991 Oct;34(10):1218–1227. doi: 10.1002/art.1780341003. [DOI] [PubMed] [Google Scholar]
- Efstathiou J. A., Noda M., Rowan A., Dixon C., Chinery R., Jawhari A., Hattori T., Wright N. A., Bodmer W. F., Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L., Näthke I. S., Papkoff J., Nelson W. J. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994 Jun;125(6):1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox S., Hallett M. B., Puntis M. C., Jiang W. G. Inhibition of cancer cell motility and invasion by interleukin-12. Clin Exp Metastasis. 1995 Sep;13(5):396–404. doi: 10.1007/BF00121916. [DOI] [PubMed] [Google Scholar]
- Inomata M., Ochiai A., Akimoto S., Kitano S., Hirohashi S. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996 May 1;56(9):2213–2217. [PubMed] [Google Scholar]
- Jou T. S., Stewart D. B., Stappert J., Nelson W. J., Marrs J. A. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci U S A. 1995 May 23;92(11):5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Lochter A., Galosy S., Muschler J., Freedman N., Werb Z., Bissell M. J. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997 Dec 29;139(7):1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logel J., Dill D., Leonard S. Synthesis of cRNA probes from PCR-generated DNA. Biotechniques. 1992 Oct;13(4):604–610. [PubMed] [Google Scholar]
- Mareel M., Bracke M., Van Roy F. Invasion promoter versus invasion suppressor molecules: the paradigm of E-cadherin. Mol Biol Rep. 1994 Jan;19(1):45–67. doi: 10.1007/BF00987321. [DOI] [PubMed] [Google Scholar]
- Mielants H., Veys E. M., Cuvelier C., de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27 (Suppl 2):95–105. doi: 10.1093/rheumatology/xxvii.suppl_2.95. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Biancone L., Marasco R., Morrone G., Marasco O., Luzza F., Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997 Apr;112(4):1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988 Dec 1;7(12):3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke I. S., Hinck L., Swedlow J. R., Papkoff J., Nelson W. J. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994 Jun;125(6):1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M., Ringwald M., Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Romagnani P., Annunziato F., Sampognaro S., Becchio A., Giannarini L., Maggi E., Pupilli C., Tonelli F., Romagnani S. Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol. 1997 Mar;150(3):823–832. [PMC free article] [PubMed] [Google Scholar]
- Poulsom R., Chinery R., Sarraf C., Lalani E. N., Stamp G., Elia G., Wright N. Trefoil peptide expression in intestinal adaptation and renewal. Scand J Gastroenterol Suppl. 1992;192:17–28. doi: 10.3109/00365529209095975. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Vaalamo M., Puolakkainen P., Airola K., Parks W. C., Karjalainen-Lindsberg M. L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996 Feb;148(2):519–526. [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Pignatelli M. The molecular histology of neoplasia: the role of the cadherin/catenin complex. Histopathology. 1997 Aug;31(2):107–111. doi: 10.1046/j.1365-2559.1997.2350845.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993 Oct;5(5):806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Valizadeh A., Karayiannakis A. J., el-Hariry I., Kmiot W., Pignatelli M. Expression of E-cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120) in colorectal polyps. Am J Pathol. 1997 Jun;150(6):1977–1984. [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]


