Abstract
OBJECTIVES—The interaction between the activation induced surface glycoprotein CD40L (ligand) (CD154) on CD4+ T cells and its receptor CD40, which is expressed on various cell types, plays a crucial part in numerous cell mediated and humoral immune reactions that may be of pathogenetic importance in rheumatoid arthritis (RA). To further evaluate the pathogenetic role of CD40L in RA, expression of CD40L and various other T cell activation antigens as well as costimulatory molecules was investigated on CD4+ T cells in RA by flow cytometry. METHODS—Two colour flow cytometry was used to determine the percentage of CD4+ T cells expressing CD40L, CD69, CD25, HLA-DR, CD39, CD27 and CD28 in peripheral blood (PB) of 62 RA patients in comparison to 20 healthy controls (HC). Disease activity was assessed by clinical, laboratory and radiological examination. Status of clinical remission of RA was evaluated according to the ACR preliminary criteria for complete clinical remission of RA. RESULTS—CD40L was expressed on > 10% of CD4+ T cells in 29% of RA patients thus defining a CD40Lhigh+ patient group. Disease activity as estimated by C reactive protein, rheumatoid factor and status of clinical remission of disease (p = 0.049) was higher in this subgroup than in the RA CD40Llow+ group. Expression of CD69, CD25, and HLA-DR was significantly increased in both RA patient groups in comparison with HC. However, the percentage of CD39+ CD4+ T cells was increased only in the RA CD40Lhigh+ subgroup (versus HC p = 0.019, versus RA CD40Llow+ p = 0.044). Furthermore, expression of CD40L and CD39 on CD4+ T cells correlated positively as estimated by Spearman rank correlation (p < 0.001). The percentage of CD4+ T cells lacking the costimulatory molecules CD27 (p = 0.002) and CD28 (p = 0.026) was increased in RA CD40Llow+ patients in comparison with HC. CONCLUSIONS—These data suggest that increased expression of CD40L on CD4+ T cells in RA indicates prolonged and increased activation of CD4+ T lymphocytes and is associated with active disease and possibly an unfavourable prognosis. Whether this phenotypically defined RA CD40Lhigh+ subgroup will preferentially respond to an anti-CD40L antibody treatment remains to be elucidated.
Full Text
The Full Text of this article is available as a PDF (148.0 KB).
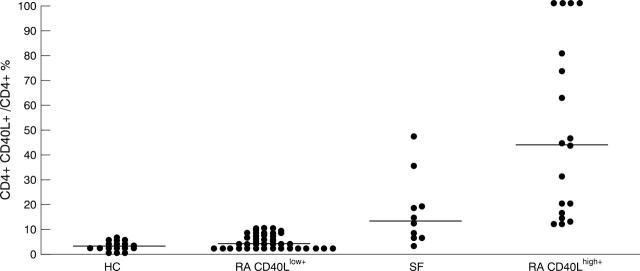
Figure 1 .
Percentage of PB CD4+ T cells expressing CD40L in healthy controls (HC, n = 20), RA CD40Llow+ (n = 44) and RA CD40Lhigh+ (n = 18) patients as well as percentage of SF CD4+ CD40L+ T cells in RA patients (SF, n = 10). The bars indicate the medians.
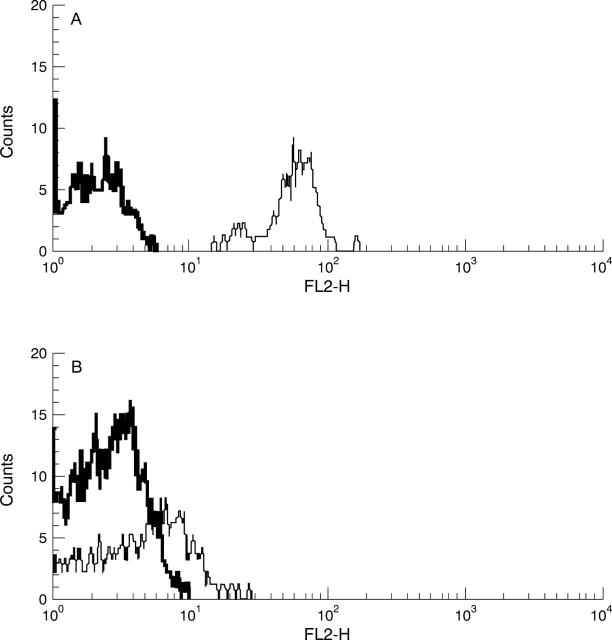
Figure 2 .
Fluorescence activated cell sorter histograms of CD40L expression (gating on CD4+ T cells) demonstrating the intensity and density of CD40L antigen expression patterns (thin line) defined as CD40Lhigh+ (A) and CD40Llow+ (B) expression. The negative control was obtained by using a isotype monoclonal antibody (thick line).
Figure 3 .
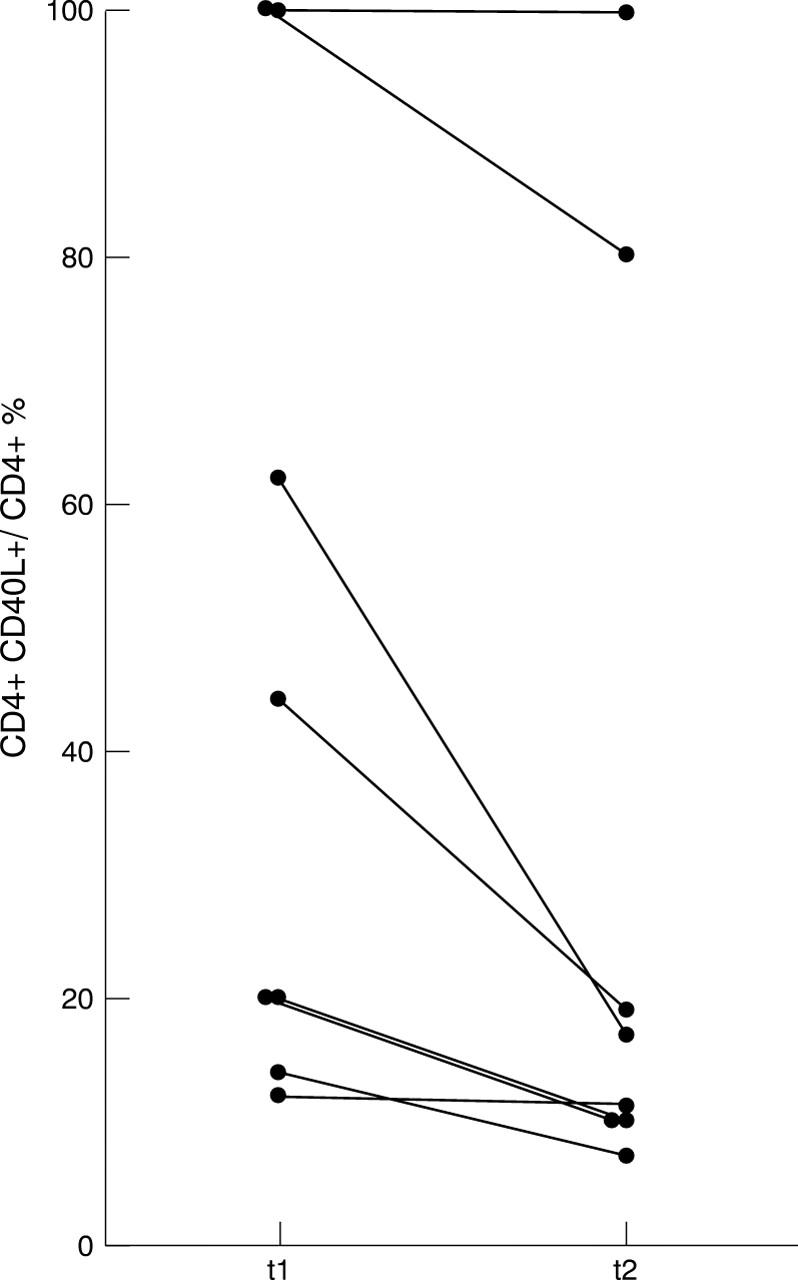
Intraindividual variability of CD40L expression in 8/18 RA CD40Lhigh+ patients. Under intensified immunosuppressive treatment the percentage of CD40L+ CD4+ T cells declined in seven of eight patients, but remained in seven of eight patients > 10%. The mean time (t1-t2) was eight months (ranging from 3 to 20 months).
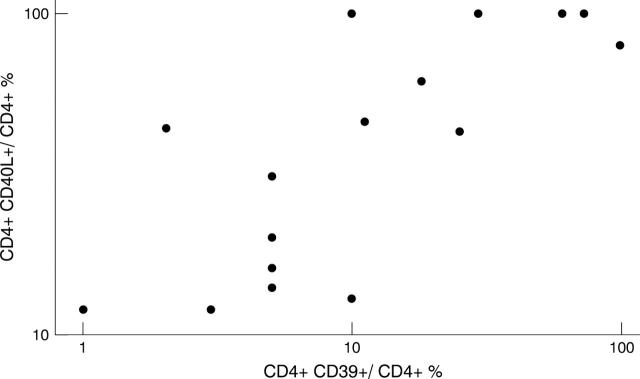
Figure 4 .
The percentage of CD39+ CD4+T cells correlated significantly (p < 0.001) with the expression of CD40L in the RA CD40Lhigh+ group as determined by Spearman rank correlation. Data are shown in logarithmic scale.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afeltra A., Galeazzi M., Sebastiani G. D., Ferri G. M., Caccavo D., Addessi M. A., Marcolongo R., Bonomo L. Coexpression of CD69 and HLADR activation markers on synovial fluid T lymphocytes of patients affected by rheumatoid arthritis: a three-colour cytometric analysis. Int J Exp Pathol. 1997 Oct;78(5):331–336. doi: 10.1046/j.1365-2613.1997.290360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Armitage R. J., Fanslow W. C., Spriggs M. K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993 Nov;14(11):559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- Clark L. B., Foy T. M., Noelle R. J. CD40 and its ligand. Adv Immunol. 1996;63:43–78. doi: 10.1016/s0065-2776(08)60854-8. [DOI] [PubMed] [Google Scholar]
- Datta S. K., Kalled S. L. CD40-CD40 ligand interaction in autoimmune disease. Arthritis Rheum. 1997 Oct;40(10):1735–1745. doi: 10.1002/art.1780401002. [DOI] [PubMed] [Google Scholar]
- Desai-Mehta A., Lu L., Ramsey-Goldman R., Datta S. K. Hyperexpression of CD40 ligand by B and T cells in human lupus and its role in pathogenic autoantibody production. J Clin Invest. 1996 May 1;97(9):2063–2073. doi: 10.1172/JCI118643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durie F. H., Fava R. A., Foy T. M., Aruffo A., Ledbetter J. A., Noelle R. J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993 Sep 3;261(5126):1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- Early G. S., Zhao W., Burns C. M. Anti-CD40 ligand antibody treatment prevents the development of lupus-like nephritis in a subset of New Zealand black x New Zealand white mice. Response correlates with the absence of an anti-antibody response. J Immunol. 1996 Oct 1;157(7):3159–3164. [PubMed] [Google Scholar]
- Fox D. A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997 Apr;40(4):598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- Gravestein L. A., Borst J. Tumor necrosis factor receptor family members in the immune system. Semin Immunol. 1998 Dec;10(6):423–434. doi: 10.1006/smim.1998.0144. [DOI] [PubMed] [Google Scholar]
- Grewal I. S., Flavell R. A. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Harigai M., Hara M., Nakazawa S., Fukasawa C., Ohta S., Sugiura T., Inoue K., Kashiwazaki S. Ligation of CD40 induced tumor necrosis factor-alpha in rheumatoid arthritis: a novel mechanism of activation of synoviocytes. J Rheumatol. 1999 May;26(5):1035–1043. [PubMed] [Google Scholar]
- Hovdenes J., Gaudernack G., Kvien T. K., Egeland T. Expression of activation markers on CD4+ and CD8+ cells from synovial fluid, synovial tissue, and peripheral blood of patients with inflammatory arthritides. Scand J Immunol. 1989 Jun;29(6):631–639. doi: 10.1111/j.1365-3083.1989.tb01167.x. [DOI] [PubMed] [Google Scholar]
- Hsu Y. M., Lucci J., Su L., Ehrenfels B., Garber E., Thomas D. Heteromultimeric complexes of CD40 ligand are present on the cell surface of human T lymphocytes. J Biol Chem. 1997 Jan 10;272(2):911–915. doi: 10.1074/jbc.272.2.911. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Shimizu H., Yoshida M., Arimori S. Activation antigens expressed on T-cells of the peripheral blood in Sjögren's syndrome and rheumatoid arthritis. Clin Exp Rheumatol. 1990 May-Jun;8(3):243–249. [PubMed] [Google Scholar]
- Kansas G. S., Wood G. S., Tedder T. F. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991 Apr 1;146(7):2235–2244. [PubMed] [Google Scholar]
- Karpusas M., Hsu Y. M., Wang J. H., Thompson J., Lederman S., Chess L., Thomas D. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995 Oct 15;3(10):1031–1039. doi: 10.1016/s0969-2126(01)00239-8. [DOI] [PubMed] [Google Scholar]
- Kohem C. L., Brezinschek R. I., Wisbey H., Tortorella C., Lipsky P. E., Oppenheimer-Marks N. Enrichment of differentiated CD45RBdim,CD27- memory T cells in the peripheral blood, synovial fluid, and synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum. 1996 May;39(5):844–854. doi: 10.1002/art.1780390518. [DOI] [PubMed] [Google Scholar]
- Koshy M., Berger D., Crow M. K. Increased expression of CD40 ligand on systemic lupus erythematosus lymphocytes. J Clin Invest. 1996 Aug 1;98(3):826–837. doi: 10.1172/JCI118855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lens S. M., Tesselaar K., van Oers M. H., van Lier R. A. Control of lymphocyte function through CD27-CD70 interactions. Semin Immunol. 1998 Dec;10(6):491–499. doi: 10.1006/smim.1998.0154. [DOI] [PubMed] [Google Scholar]
- MacDonald K. P., Nishioka Y., Lipsky P. E., Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997 Nov 1;100(9):2404–2414. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maliszewski C. R., Delespesse G. J., Schoenborn M. A., Armitage R. J., Fanslow W. C., Nakajima T., Baker E., Sutherland G. R., Poindexter K., Birks C. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994 Oct 15;153(8):3574–3583. [PubMed] [Google Scholar]
- Martens P. B., Goronzy J. J., Schaid D., Weyand C. M. Expansion of unusual CD4+ T cells in severe rheumatoid arthritis. Arthritis Rheum. 1997 Jun;40(6):1106–1114. doi: 10.1002/art.1780400615. [DOI] [PubMed] [Google Scholar]
- Maurer D., Felzmann T., Holter W., Petera P., Smolen J., Knapp W. Evidence for the presence of activated CD4 T cells with naive phenotype in the peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1992 Mar;87(3):429–434. doi: 10.1111/j.1365-2249.1992.tb03014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan C., Shi Y., Laman J. D., Datta S. K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995 Feb 1;154(3):1470–1480. [PubMed] [Google Scholar]
- Müller B., Gimsa U., Mitchison N. A., Radbruch A., Sieper J., Yin Z. Modulating the Th1/Th2 balance in inflammatory arthritis. Springer Semin Immunopathol. 1998;20(1-2):181–196. doi: 10.1007/BF00832006. [DOI] [PubMed] [Google Scholar]
- Namekawa T., Wagner U. G., Goronzy J. J., Weyand C. M. Functional subsets of CD4 T cells in rheumatoid synovitis. Arthritis Rheum. 1998 Dec;41(12):2108–2116. doi: 10.1002/1529-0131(199812)41:12<2108::AID-ART5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Noelle R. J., Roy M., Shepherd D. M., Stamenkovic I., Ledbetter J. A., Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinals R. S., Baum J., Bland J., Fosdick W. M., Kaplan S. B., Masi A. T., Mitchell D. M., Ropes M. W., Short C. L., Sigler J. W. Preliminary criteria for clinical remission in rheumatoid arthritis. Bull Rheum Dis. 1982;32(1):7–10. [PubMed] [Google Scholar]
- Schmidt D., Goronzy J. J., Weyand C. M. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996 May 1;97(9):2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel L. A., Noelle R. J. CD40 and its crucial role as a member of the TNFR family. Semin Immunol. 1998 Dec;10(6):435–442. doi: 10.1006/smim.1998.0145. [DOI] [PubMed] [Google Scholar]
- Wang T. F., Guidotti G. CD39 is an ecto-(Ca2+,Mg2+)-apyrase. J Biol Chem. 1996 Apr 26;271(17):9898–9901. [PubMed] [Google Scholar]
- Yellin M. J., Winikoff S., Fortune S. M., Baum D., Crow M. K., Lederman S., Chess L. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J Leukoc Biol. 1995 Aug;58(2):209–216. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]