Abstract
OBJECTIVE—To determine whether the reaction of rheumatoid factor (RF) with solid phase histone is due to the simultaneous presence of circulating immune complexes (CICs) or aggregated IgG. METHODS—Serum samples from 56 patients with seropositive rheumatoid arthritis (RA) and 50 random blood bank donors were used. Binding of immunoglobulins to histone was determined by enzyme linked immunosorbent assay (ELISA) and by western blots. Aggregated IgG was obtained by heating at 61oC for 30 minutes. RESULTS—Among the RA sera tested by ELISA, 54% were positive for histone binding by IgM, IgG, or IgA and 20% by IgM only. Heating of normal sera caused a significant enhancement in the binding of IgG to histone (p<0.001). This binding had a non-cognate behaviour—that is, it was destroyed by pepsin treatment of serum and was not significantly inhibited by competition with free histone. The same behaviour was seen for IgM, IgG, and IgA binding from RA sera. However, cognate IgG antibody binding to histone was inhibited by free histone and was resistant to pepsin digestion. Addition of heat aggregated IgG to RA sera or pretreatment of histone with aggregated IgG caused a significant increase in IgM binding to histone. CONCLUSION—IgM, IgG, and IgA RF bind to solid phase histone as a result of attachment to histone of immune complexes or aggregated IgG and not as a result of a cognate reaction with histone.
Full Text
The Full Text of this article is available as a PDF (174.9 KB).
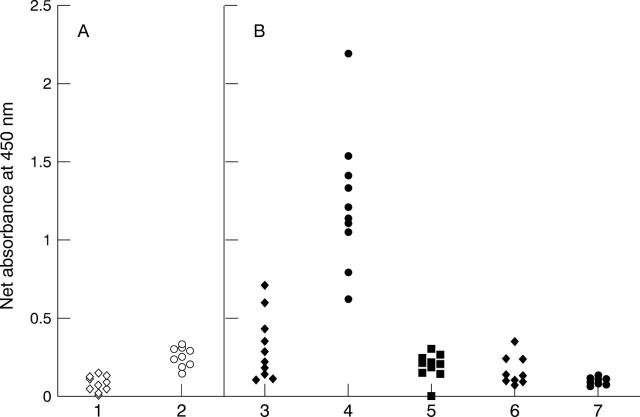
Figure 1 .
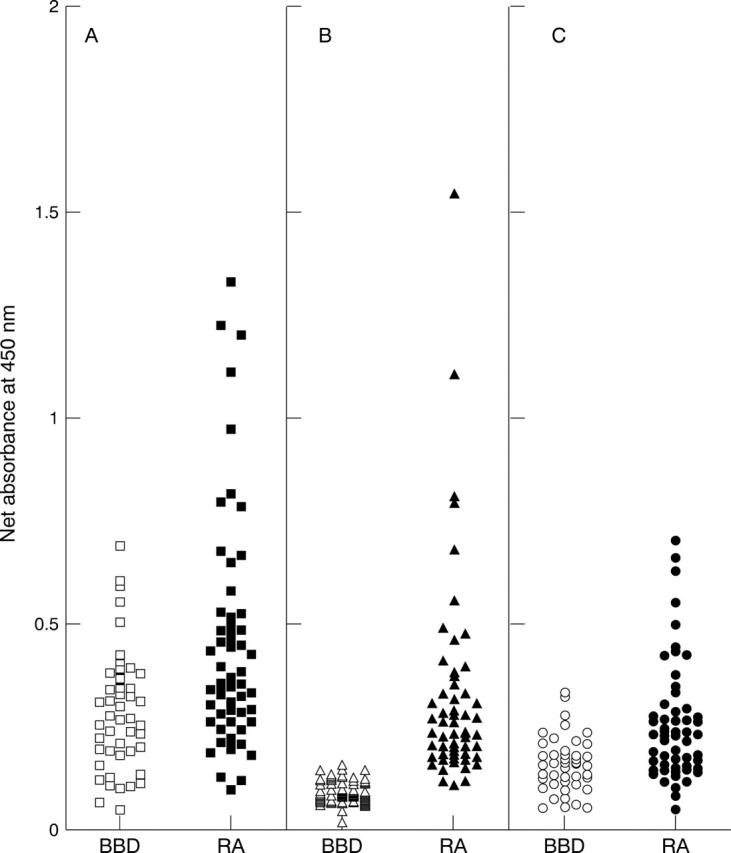
Reaction with solid phase histone of immunoglobulin from control and RA sera. Scattergram of the net A450 values yielded by ELISA on histone coated wells, reacted with sera from 50 blood bank donors (BBD, open symbols) and 56 patients with rheumatoid arthritis (RA, solid symbols). Enzyme-conjugate was isotype-specific anti-IgM (A), anti-IgG (B), or anti-IgA (C).
Figure 2 .
Effect of serum heating at 61°C on the binding of IgM RF from RA sera to histone and to other nuclear antigens. Scattergram of the net A450 yielded on ELISA by serum samples from blood bank donors (A) and patients with rheumatoid arthritis sera (B): non-heated (1, 3) or heated at 61°C for 30 minutes (2, 4, 5, 6, 7). Wells were coated with histones (1-4), ssDNA (5), nRNP/Sm (6) or were blanks (7, raw A450). Isotype-specific enzyme-conjugate was anti-IgM.
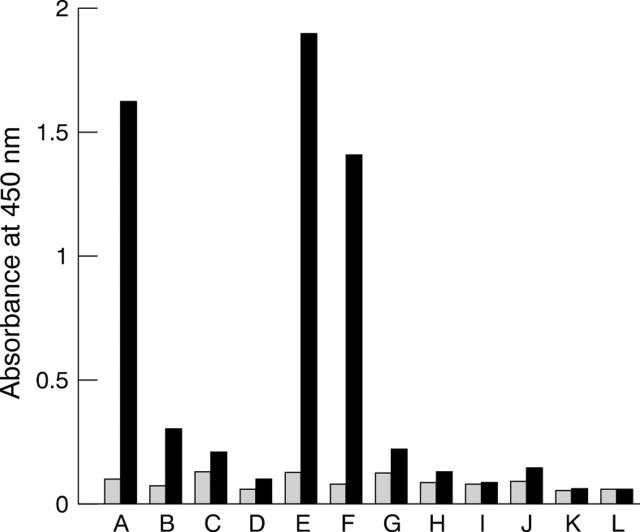
Figure 3 .
Effect of the pretreatment of histone coated wells with aggregated serum IgG on the binding to histone of IgM from RA sera. A450 yielded by blank plates (shaded bars) and by wells coated with histone (solid bars) of normal serum heated at 61°C for 30 minutes (A, B); non-heated RA serum (C, D); RA serum on wells preincubated for 30 minutes with heated normal serum (E, F) or non-heated normal serum (G, H); and by non-heated normal serum (I, J), and enzyme conjugates (K, L) as controls. RA serum was diluted 1/100 (C, E, G), or 1/1000 (D, F, H). Reactions were shown with isotype-specific enzyme-antibody conjugates: goat antihuman Fcγ chain-specific (lanes A, I, K), or rabbit antihuman Fc5µ (lanes B, C, D, E, F, G, H, J, L) antibodies.
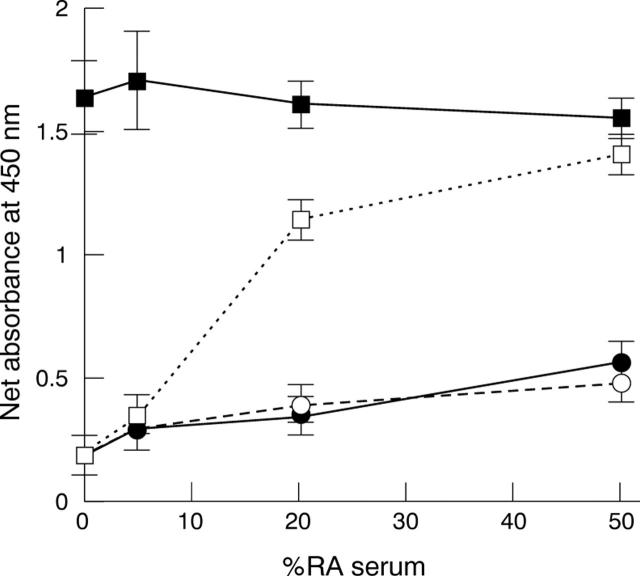
Figure 4 .
Effect of addition of heat aggregated serum IgG to RA serum on the binding to histone of IgM. Net A450 (SD) yielded by mixtures of RA serum and non-heated normal serum (circles), and RA serum and heated normal serum (squares). The proportion of RA serum in the mix was variable (%RA serum). Binding was shown by anti-IgG enzyme-conjugate (solid symbols, solid line) or anti-IgM (open symbols, dashed line).
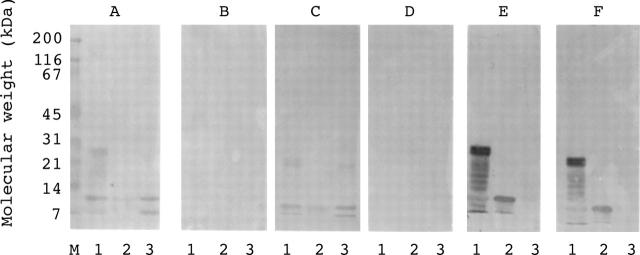
Figure 5 .
Effect of pepsin digestion on the binding of heat aggregated IgG and of antihistone antibodies to histone as determined by western blots. Proteins on the membrane were prestained molecular weight standards (lane M); a mix of all histone proteins (lane 1); H2A and H2B (lane 2); H3 and H4 (lane 3). The different panels were reacted with different primary reagents: heated normal serum (panel A); heated normal serum subjected to pepsin digestion (panel B); RA serum (specimen R27) (panel C); RA serum subjected to pepsin digestion (panel D); serum containing antihistone antibody (spiked specimen HS-1) (panel E); and serum containing antihistone antibody subjected to pepsin digestion (panel F).
Figure 6 .
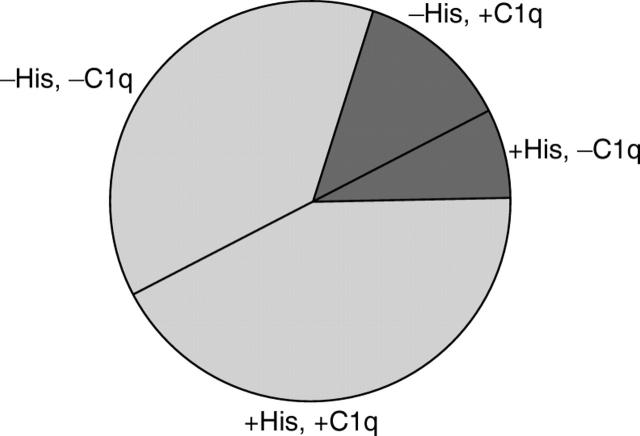
Proportion of 40 RA serum samples with IgG that reacted with C1q or with histone, or both in ELISA. The darker zones represent discrepant specimens; the lighter overlap zones represent the samples positive or negative for both C1q and histone.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Chakraborty G., Hajra B., Bhattacharya S., Sikdar P. K., Sinha S., Banerjee P. P., Ghosh E., Chakraborty P. Serology and immunoglobulin profile in rheumatoid arthritis. Indian J Pathol Microbiol. 1998 Jan;41(1):43–47. [PubMed] [Google Scholar]
- Agnello V., Arbetter A., Ibanez de Kasep G., Powell R., Tan E. M., Joslin F. Evidence for a subset of rheumatoid factors that cross-react with DNA-histone and have a distinct cross-idiotype. J Exp Med. 1980 Jun 1;151(6):1514–1527. doi: 10.1084/jem.151.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreis M., Hurd E. R., Lospalluto J., Ziff M. Comparison of the presence of immune complexes in Felty's syndrome and rheumatoid arthritis. Arthritis Rheum. 1978 Apr;21(3):310–315. doi: 10.1002/art.1780210304. [DOI] [PubMed] [Google Scholar]
- Axford J. S., Sumar N., Alavi A., Isenberg D. A., Young A., Bodman K. B., Roitt I. M. Changes in normal glycosylation mechanisms in autoimmune rheumatic disease. J Clin Invest. 1992 Mar;89(3):1021–1031. doi: 10.1172/JCI115643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. A., Anderson B. E. An immune complex selective affinity matrix utilizing a synthetic peptide. J Biol Chem. 1990 Oct 25;265(30):18414–18422. [PubMed] [Google Scholar]
- Bernstein K. A., Valerio R. D., Lefkowith J. B. Glomerular binding activity in MRL lpr serum consists of antibodies that bind to a DNA/histone/type IV collagen complex. J Immunol. 1995 Mar 1;154(5):2424–2433. [PubMed] [Google Scholar]
- Bustos A., Boimorto R., Subiza J. L., Pereira L. F., Marco M., Figueredo M. A., de la Concha E. G. Inhibition of histone/anti-histone reactivity by histone-binding serum components; differential effect on anti-H1 versus anti-H2B antibodies. Clin Exp Immunol. 1994 Mar;95(3):408–414. doi: 10.1111/j.1365-2249.1994.tb07011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrespines A., Laderach D., Lebossé C., Bach J. F., Koutouzov S. Isolation and characterization of apoptotic nucleosomes, free and complexed with lupus autoantibody generated during hybridoma B-cell apoptosis. J Autoimmun. 1998 Feb;11(1):19–27. doi: 10.1006/jaut.1997.0172. [DOI] [PubMed] [Google Scholar]
- Cohen M. G., Webb J. Antihistone antibodies in rheumatoid arthritis and Felty's syndrome. Arthritis Rheum. 1989 Oct;32(10):1319–1324. doi: 10.1002/anr.1780321020. [DOI] [PubMed] [Google Scholar]
- Csepregi A., Nemesánszky E., Bély M. Kryoglobulinämie und chronische Lebererkrankungen. Z Gastroenterol. 1998 May;36(5):391–401. [PubMed] [Google Scholar]
- De Bandt M., Meyer O. Manifestations extra-articulaires de la polyarthrite rhumatoïde. Rev Prat. 1997 Nov 15;47(18):2012–2016. [PubMed] [Google Scholar]
- Fornasieri A., D'Amico G. Type II mixed cryoglobulinaemia, hepatitis C virus infection, and glomerulonephritis. Nephrol Dial Transplant. 1996;11 (Suppl 4):25–30. doi: 10.1093/ndt/11.supp4.25. [DOI] [PubMed] [Google Scholar]
- Hannestad K., Rekvig O. P., Husebekk A. Cross-reacting rheumatoid factors and lupus erythematosus (LE)-factors. Springer Semin Immunopathol. 1981;4(2):133–160. doi: 10.1007/BF01857092. [DOI] [PubMed] [Google Scholar]
- Hurd E. R., Chubick A., Jasin H. E., Ziff M. Increased C1q binding immune complexes in Felty's syndrome: comparison with uncomplicated rheumatoid arthritis. Arthritis Rheum. 1979 Jul;22(7):697–702. doi: 10.1002/art.1780220702. [DOI] [PubMed] [Google Scholar]
- Konikoff F., Swissa M., Shoenfeld Y. Autoantibodies to histones and their subfractions in chronic liver diseases. Clin Immunol Immunopathol. 1989 Apr;51(1):77–82. doi: 10.1016/0090-1229(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Muzellec Y., Le Goff P., Jouquan J., Fauquert P., Muller S., Youinou P. Antibodies to histones in rheumatoid arthritis. Diagn Clin Immunol. 1988;5(6):326–331. [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Nucleosomes: the structural quantum in chromosomes. Am Sci. 1978 Nov-Dec;66(6):704–711. [PubMed] [Google Scholar]
- Pasquali J. L., Azerad G., Martin T., Muller S. The double reactivity of a human monoclonal rheumatoid factor to IgG and histones is related to distinct binding sites. Eur J Immunol. 1988 Jul;18(7):1127–1130. doi: 10.1002/eji.1830180724. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Balderas R. S., Tan E. M., Dixon F. J., Theofilopoulos A. N. Multiple autoantigen binding capabilities of mouse monoclonal antibodies selected for rheumatoid factor activity. J Exp Med. 1984 May 1;159(5):1429–1440. doi: 10.1084/jem.159.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedler W., Wallman J., Froelich C. J., Teodorescu M. Routine measurement of IgM, IgG, and IgA rheumatoid factors: high sensitivity, specificity, and predictive value for rheumatoid arthritis. J Rheumatol. 1997 Jun;24(6):1037–1044. [PubMed] [Google Scholar]
- Tax W. J., Kramers C., van Bruggen M. C., Berden J. H. Apoptosis, nucleosomes, and nephritis in systemic lupus erythematosus. Kidney Int. 1995 Sep;48(3):666–673. doi: 10.1038/ki.1995.336. [DOI] [PubMed] [Google Scholar]
- Termaat R. M., Brinkman K., van Gompel F., van den Heuvel L. P., Veerkamp J. H., Smeenk R. J., Berden J. H. Cross-reactivity of monoclonal anti-DNA antibodies with heparan sulfate is mediated via bound DNA/histone complexes. J Autoimmun. 1990 Oct;3(5):531–545. doi: 10.1016/s0896-8411(05)80019-8. [DOI] [PubMed] [Google Scholar]
- van der Giessen M., The T. H. Characterization of the soluble immune complexes that are detected by three different techniques. Clin Immunol Immunopathol. 1986 Feb;38(2):244–255. doi: 10.1016/0090-1229(86)90142-x. [DOI] [PubMed] [Google Scholar]