Abstract
OBJECTIVE—To determine the evolution of levels of total serum ferritin and percentage of the glycosylated form in patients with adult onset Still's disease (AOSD) at the time of diagnosis and during follow up. METHODS—All patients with AOSD were tested at the time of diagnosis and during follow up. Total serum ferritin levels were analysed by immunoassay, and the percentage of glycosylated ferritin was determined by methods using Sepharose-Con A. RESULTS—14 patients (eight women, six men) with AOSD were enrolled. At the time of diagnosis, mean (SD) age was 36 (16) years. Mean initial total serum ferritin was 6350 (1300) µg/l (normal <250 µg/l). The mean initial percentage of glycosylated ferritin was 14.7 (13)% (normal >50%). Mean follow up time was 37 (35) months. At the time of the last examination all patients were in remission except one, who presented a chronic articular form. Total serum ferritin remained high in this single patient and was normal in the 13 others, with a mean of 98 (73) µg/l. In all patients the percentage of glycosylated ferritin remained low, with a mean of 16 (16)%. CONCLUSION—Total serum ferritin is a marker of the active phase of AOSD. The percentage of glycosylated ferritin is low both in the active phase and in remission. Further studies are needed to confirm these data and to determine their specificity for AOSD before considering any possible use of a low percentage of glycosylated ferritin as a diagnostic tool in suspected AOSD, especially when atypical or previously treated.
Full Text
The Full Text of this article is available as a PDF (113.1 KB).
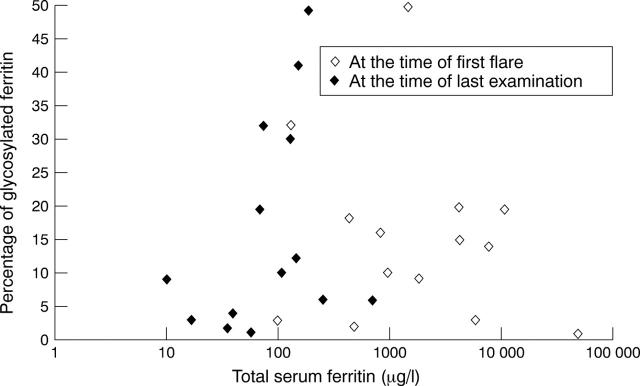
Figure 1 .
Percentage of glycosylated ferritin and total serum ferritin at the time of diagnosis and during follow up. No correlation was observed between the percentage of glycosylated ferritin and total serum ferritin (r = −0.19, NS).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Basset C., Dueymes M., Devauchelle V., Mimassi N. G., Pennec Y. L., Youinou P. Changes in glycosylation of immunoglobulins in primary Sjögren's syndrome. Ann Med Interne (Paris) 1998 Feb;149(1):42–44. [PubMed] [Google Scholar]
- Cazzola M., Borgna-Pignatti C., de Stefano P., Bergamaschi G., Bongo I. G., Dezza L., Avato F. Internal distribution of excess iron and sources of serum ferritin in patients with thalassemia. Scand J Haematol. 1983 Apr;30(4):289–296. doi: 10.1111/j.1600-0609.1983.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Cragg S. J., Wagstaff M., Worwood M. Sialic acid and the microheterogeneity of human serum ferritin. Clin Sci (Lond) 1980 Mar;58(3):259–262. doi: 10.1042/cs0580259. [DOI] [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T., Martin-Mola E., Fernandez-Zamorano A., Balsa-Criado A., de Miguel-Mendieta E. Serum ferritin can be useful for diagnosis in adult onset Still's disease. J Rheumatol. 1989 Mar;16(3):412–413. [PubMed] [Google Scholar]
- Higashi S., Ota T., Eto S. Biochemical analysis of ferritin subunits in sera from adult Still's disease patients. Rheumatol Int. 1995;15(2):45–50. doi: 10.1007/BF00262707. [DOI] [PubMed] [Google Scholar]
- Ohta A., Yamaguchi M., Kaneoka H., Nagayoshi T., Hiida M. Adult Still's disease: review of 228 cases from the literature. J Rheumatol. 1987 Dec;14(6):1139–1146. [PubMed] [Google Scholar]
- Ota T., Higashi S., Suzuki H., Eto S. Increased serum ferritin levels in adult Still's disease. Lancet. 1987 Mar 7;1(8532):562–563. doi: 10.1016/s0140-6736(87)90204-2. [DOI] [PubMed] [Google Scholar]
- Schwarz-Eywill M., Heilig B., Bauer H., Breitbart A., Pezzutto A. Evaluation of serum ferritin as a marker for adult Still's disease activity. Ann Rheum Dis. 1992 May;51(5):683–685. doi: 10.1136/ard.51.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth C., Le Moel G., Lasne Y., Revenant M. C., Agneray J., Kahn M. F., Bourgeois P. Serum ferritin and isoferritins are tools for diagnosis of active adult Still's disease. J Rheumatol. 1994 May;21(5):890–895. [PubMed] [Google Scholar]
- Vignes S., Wechsler B., Amoura Z., Papo T., Francès C., Huong D. L., Veyssier P., Godeau P., Piette J. C. Intravenous immunoglobulin in adult Still's disease refractory to non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol. 1998 May-Jun;16(3):295–298. [PubMed] [Google Scholar]
- Worwood M., Cragg S. J., Wagstaff M., Jacobs A. Binding of human serum ferritin to concanavalin A. Clin Sci (Lond) 1979 Jan;56(1):83–87. doi: 10.1042/cs0560083. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Ohta A., Tsunematsu T., Kasukawa R., Mizushima Y., Kashiwagi H., Kashiwazaki S., Tanimoto K., Matsumoto Y., Ota T. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992 Mar;19(3):424–430. [PubMed] [Google Scholar]