Abstract
OBJECTIVES—Assessment of the numbers and spatial distribution of cells producing interleukin 1α (IL1α), interleukin 1β (IL1β), tumour necrosis factor α (TNFα), and interleukin 6 (IL6) in the synovial membranes of patients with rheumatoid arthritis (RA). METHODS—Synovial tissue specimens from 40 patients with RA and eight patients with non-rheumatic disease were obtained by arthroscopy guided biopsy techniques or during joint surgery. A modified immunohistochemical method detecting cytokine producing rather than cytokine binding cells was applied to determine cytokine synthesis in fixed cryopreserved sections. Computerised image analysis methods provided comparative quantitative assessments. RESULTS—A wide variation between subjects was recorded for both quantities and profiles of expressed cytokines, despite similar macroscopic and histopathological features of inflammation. IL1α and IL1β were the most abundant monokines identified, though produced at different sites. IL1α was predominantly seen in vascular endothelial cells, whereas IL1β staining was mainly shown in macrophages and fibroblasts. Concordant results for the detection of TNFα at protein and mRNA levels were obtained with an unexpectedly low number of TNFα producing cells compared with IL1 expressing cells in many patients with RA. Specimens acquired arthroscopically from areas with maximum signs of macroscopic inflammation showed an increased number of TNFα producing cells in pannus tissue compared with that occurring in synovial villi of a given joint. This clustered distribution was not found for cells expressing any of the other studied cytokines. CONCLUSION—The recorded heterogeneous profile of proinflammatory cytokine synthesis in the synovial membrane among patients with RA may provide a clue for an understanding of the wide variation in responsiveness to different modes of antirheumatic treatment between patients.
Full Text
The Full Text of this article is available as a PDF (206.4 KB).
Figure 1 .
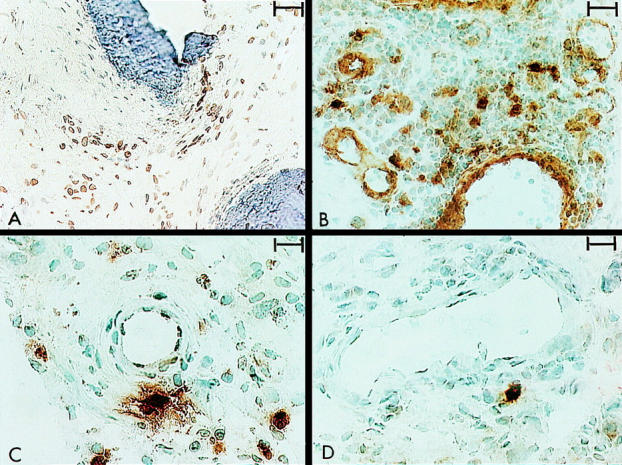
Videoprint photographs illustrating brown (diaminobenzidine) immunoperoxidase staining for cytokine producing cells in cryopreserved synovial membrane biopsy specimens obtained from patients with rheumatoid arthritis (RA). The cells were counterstained with haematoxylin. (A) Interleukin 1β producing cells in pannus tissue penetrating bone (dark blue staining) (original magnification ×200, bar represents 40 µm). (B) Interleukin 1α production occurring in both vascular endothelial cells and individual macrophages (original magnification ×320, bar represents 32 µm). (C) Tumour necrosis factor α (TNFα) producing cells in the sublining layer with additional extracellular TNFα staining encompassing producer cells (original magnification ×400, bar represents 20 µm). (D) Interleukin 6 production was seen in scattered cells with minimum additional extracellular staining (original magnification ×400, bar represents 20 µm).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Brown C. B., Kaushansky K., Firestein G. S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991 May 15;146(10):3365–3371. [PubMed] [Google Scholar]
- Andersson J., Abrams J., Björk L., Funa K., Litton M., Agren K., Andersson U. Concomitant in vivo production of 19 different cytokines in human tonsils. Immunology. 1994 Sep;83(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Arvidson N. G., Gudbjörnsson B., Elfman L., Rydén A. C., Tötterman T. H., Hällgren R. Circadian rhythm of serum interleukin-6 in rheumatoid arthritis. Ann Rheum Dis. 1994 Aug;53(8):521–524. doi: 10.1136/ard.53.8.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan F. M., Chantry D., Jackson A., Maini R., Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989 Jul 29;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Field M., Chu C. Q., Feldmann M., Maini R. N. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30 (Suppl 1):76–80. [PubMed] [Google Scholar]
- Brennan F. M., Zachariae C. O., Chantry D., Larsen C. G., Turner M., Maini R. N., Matsushima K., Feldmann M. Detection of interleukin 8 biological activity in synovial fluids from patients with rheumatoid arthritis and production of interleukin 8 mRNA by isolated synovial cells. Eur J Immunol. 1990 Sep;20(9):2141–2144. doi: 10.1002/eji.1830200938. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Cunnane G., Youssef P., Yanni G., Fitzgerald O., Mulherin D. Microscopic measurement of synovial membrane inflammation in rheumatoid arthritis: proposals for the evaluation of tissue samples by quantitative analysis. Br J Rheumatol. 1998 Jun;37(6):636–642. doi: 10.1093/rheumatology/37.6.636. [DOI] [PubMed] [Google Scholar]
- Buchan G., Barrett K., Turner M., Chantry D., Maini R. N., Feldmann M. Interleukin-1 and tumour necrosis factor mRNA expression in rheumatoid arthritis: prolonged production of IL-1 alpha. Clin Exp Immunol. 1988 Sep;73(3):449–455. [PMC free article] [PubMed] [Google Scholar]
- Cauli A., Yanni G., Panayi G. S. Interleukin-1, interleukin-1 receptor antagonist and macrophage populations in rheumatoid arthritis synovial membrane. Br J Rheumatol. 1997 Sep;36(9):935–940. doi: 10.1093/rheumatology/36.9.935. [DOI] [PubMed] [Google Scholar]
- Cañete J. D., Llena J., Collado A., Sanmartí R., Gayá A., Gratacós J., Blay M., Muñoz-Gómez J. Comparative cytokine gene expression in synovial tissue of early rheumatoid arthritis and seronegative spondyloarthropathies. Br J Rheumatol. 1997 Jan;36(1):38–42. doi: 10.1093/rheumatology/36.1.38. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Allard S., Abney E., Feldmann M., Maini R. N. Detection of cytokines at the cartilage/pannus junction in patients with rheumatoid arthritis: implications for the role of cytokines in cartilage destruction and repair. Br J Rheumatol. 1992 Oct;31(10):653–661. doi: 10.1093/rheumatology/31.10.653. [DOI] [PubMed] [Google Scholar]
- Chu C. Q., Field M., Feldmann M., Maini R. N. Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1125–1132. doi: 10.1002/art.1780340908. [DOI] [PubMed] [Google Scholar]
- Cunnane G., Bjork L., Ulfgren A. K., Lindblad S., FitzGerald O., Bresnihan B., Andersson U. Quantitative analysis of synovial membrane inflammation: a comparison between automated and conventional microscopic measurements. Ann Rheum Dis. 1999 Aug;58(8):493–499. doi: 10.1136/ard.58.8.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Katsikis P., Feldmann M., Maini R. N. Localization of interleukin-1 alpha, type 1 interleukin-1 receptor and interleukin-1 receptor antagonist in the synovial membrane and cartilage/pannus junction in rheumatoid arthritis. Br J Rheumatol. 1992 Dec;31(12):801–809. doi: 10.1093/rheumatology/31.12.801. [DOI] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Mitchell T., Feldmann M., Maini R. N. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992 Oct;35(10):1170–1178. doi: 10.1002/art.1780351009. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Cytokine Growth Factor Rev. 1997 Dec;8(4):253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- Dolhain R. J., Andersson U., ter Haar N. T., Brinkman B. M., Verweij C. L., Daha M. R., Breedveld F. C., Miltenburg A. M. Detection of intracellular interferon-gamma by light microscopy using an immunoperoxidase technique: correlation with the corresponding mRNA and protein product. J Leukoc Biol. 1993 Dec;54(6):545–551. doi: 10.1002/jlb.54.6.545. [DOI] [PubMed] [Google Scholar]
- Eastgate J. A., Symons J. A., Wood N. C., Grinlinton F. M., di Giovine F. S., Duff G. W. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988 Sep 24;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Farahat M. N., Yanni G., Poston R., Panayi G. S. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993 Dec;52(12):870–875. doi: 10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M., Brennan F., Paleolog E., Taylor P., Maini R. N. Anti-tumor necrosis factor alpha therapy of rheumatoid arthritis. Mechanism of action. Eur Cytokine Netw. 1997 Sep;8(3):297–300. [PubMed] [Google Scholar]
- Field M., Chu C., Feldmann M., Maini R. N. Interleukin-6 localisation in the synovial membrane in rheumatoid arthritis. Rheumatol Int. 1991;11(2):45–50. doi: 10.1007/BF00291144. [DOI] [PubMed] [Google Scholar]
- Henter J. I., Söder O., Andersson U. Identification of individual tumor necrosis factor/cachectin-producing cells after lipopolysaccharide induction. Eur J Immunol. 1988 Jul;18(7):983–988. doi: 10.1002/eji.1830180703. [DOI] [PubMed] [Google Scholar]
- Husby G., Williams R. C., Jr Immunohistochemical studies of interleukin-2 and gamma-interferon in rheumatoid arthritis. Arthritis Rheum. 1985 Feb;28(2):174–181. doi: 10.1002/art.1780280212. [DOI] [PubMed] [Google Scholar]
- Khademi M., Wallström E., Andersson M., Piehl F., Di Marco R., Olsson T. Reduction of both pro- and anti-inflammatory cytokines after 6 months of interferon beta-1a treatment of multiple sclerosis. J Neuroimmunol. 2000 Mar 1;103(2):202–210. doi: 10.1016/s0165-5728(99)00184-8. [DOI] [PubMed] [Google Scholar]
- Kirkham B. Interleukin-1, immune activation pathways, and different mechanisms in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis. 1991 Jun;50(6):395–400. doi: 10.1136/ard.50.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Malmnäs Tjernlund U. K., Kabelitz D., Wigren A. Appearance of anti-HLA-DR-reactive cells in normal and rheumatoid synovial tissue. Scand J Immunol. 1981 Aug;14(2):183–192. doi: 10.1111/j.1365-3083.1981.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Klimiuk P. A., Goronzy J. J., Björ nsson J., Beckenbaugh R. D., Weyand C. M. Tissue cytokine patterns distinguish variants of rheumatoid synovitis. Am J Pathol. 1997 Nov;151(5):1311–1319. [PMC free article] [PubMed] [Google Scholar]
- Lindblad S., Hedfors E. Intraarticular variation in synovitis. Local macroscopic and microscopic signs of inflammatory activity are significantly correlated. Arthritis Rheum. 1985 Sep;28(9):977–986. doi: 10.1002/art.1780280904. [DOI] [PubMed] [Google Scholar]
- Litton M. J., Dohlsten M., Hansson J., Rosendahl A., Ohlsson L., Kalland T., Andersson J., Andersson U. Tumor therapy with an antibody-targeted superantigen generates a dichotomy between local and systemic immune responses. Am J Pathol. 1997 May;150(5):1607–1618. [PMC free article] [PubMed] [Google Scholar]
- Lundberg I., Ulfgren A. K., Nyberg P., Andersson U., Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997 May;40(5):865–874. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]
- McNiff P. A., Stewart C., Sullivan J., Showell H. J., Gabel C. A. Synovial fluid from rheumatoid arthritis patients contains sufficient levels of IL-1 beta and IL-6 to promote production of serum amyloid A by Hep3B cells. Cytokine. 1995 Feb;7(2):209–219. doi: 10.1006/cyto.1995.1028. [DOI] [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Tetta C., Camussi G., Modena V., Di Vittorio C., Baglioni C. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990 Sep;49(9):665–667. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfgren A. K., Lindblad S., Klareskog L., Andersson J., Andersson U. Detection of cytokine producing cells in the synovial membrane from patients with rheumatoid arthritis. Ann Rheum Dis. 1995 Aug;54(8):654–661. doi: 10.1136/ard.54.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Fritz P., Einsele H., Sell S., Saal J. G. Evaluation of synovial cytokine patterns in rheumatoid arthritis and osteoarthritis by quantitative reverse transcription polymerase chain reaction. Rheumatol Int. 1997;16(5):191–196. doi: 10.1007/BF01330295. [DOI] [PubMed] [Google Scholar]
- Westacott C. I., Whicher J. T., Barnes I. C., Thompson D., Swan A. J., Dieppe P. A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990 Sep;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. M., Haines G. K., Shah M. R., Rayan G., Koch A. E. Low-level production of interleukin-13 in synovial fluid and tissue from patients with arthritis. Clin Immunol Immunopathol. 1997 Nov;85(2):210–220. doi: 10.1006/clin.1997.4441. [DOI] [PubMed] [Google Scholar]
- Xu W. D., Firestein G. S., Taetle R., Kaushansky K., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989 Mar;83(3):876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanni G., Whelan A., Feighery C., Quinlan W., Symons J., Duff G., Bresnihan B. Contrasting levels of in vitro cytokine production by rheumatoid synovial tissues demonstrating different patterns of mononuclear cell infiltration. Clin Exp Immunol. 1993 Sep;93(3):387–395. doi: 10.1111/j.1365-2249.1993.tb08190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


